Abstract
Background
Previous studies have investigated cardiovascular disease (CVD) risks in cancer patients, but there is limited knowledge concerning the CVD risk in adult and young adolescent (AYA) survivors of gastric cancer.
Objectives
This study aims to investigate the incidence of CVD in AYA gastric cancer survivors, analyzing it by treatment type and identifying associated risk factors.
Methods
We conducted a retrospective cohort study using Korean National Health Insurance Service data collected from 2006 to 2019. Propensity score matching (1:3, caliper < 0.1) was performed using the variables age, sex, income, residential area, and presence of comorbidities, and we classified participants into gastric cancer (n = 6562) and non-cancer control (n = 19,678) groups. Cox regression models were used to calculate hazard ratios (HRs) for CVD incidence. The study assessed CVD incidence by cancer treatment and identified risk factors through multivariable Cox regression.
Results
During a median 6.5-year follow-up, AYA gastric cancer survivors consistently exhibited greater CVD incidence. Their risk of CVD was significantly elevated compared to that of controls (HR, 1.18; 95% confidence interval [CI] 1.05–1.33). In particular, deep vein thrombosis (HR, 3.93; 95% CI 3.06–14.67) and pulmonary embolism (HR, 6.58; 95% CI 3.06–14.67) risks were notably increased. Chemotherapy was associated with an increased risk of stroke, heart failure, atrial fibrillation, deep vein thrombosis, and pulmonary embolism. Hypertension (HR, 1.58; 95% CI 1.10–2.26) and dyslipidemia (HR, 1.46; 95% CI 1.06–2.20) emerged as risk factors for CVD development.
Conclusion
This study reports elevated risks of CVD in AYA gastric cancer survivors and emphasizes the need for vigilant monitoring of CVD in this population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer remains a significant global health concern, accounting for a substantial burden of cancer-related morbidity and mortality [1]. Among affected individuals, adolescents and young adults (AYAs) represent a unique subset characterized by distinctive disease patterns and management challenges [2]. AYA gastric cancer patients often present with more advanced and aggressive pathogenesis compared to older gastric cancer patients, probably due to delayed diagnosis and inherited genetic factors [3]. [4]. While investigations into the cardiovascular disease (CVD) risk among cancer patients have been conducted in various contexts [5,6,7,8], research specifically targeting AYAs with gastric cancer is notably scarce.
Previous studies exploring the CVD risk in AYA cancer patients have reported the potential implications of cancer treatments and the long-term health outcomes of the AYA population [9,10,11]. However, most of these investigations have primarily focused on cancers that more commonly occur in Western AYAs, such as lymphoma, thyroid, or gynecologic cancer [12,13,14], with limited attention paid to gastric cancer. In addition, general population-based studies have reported a decreased risk of CVDs among older gastric cancer patients. One cohort study from Korea documented a decreased risk of coronary heart disease and ischemic stroke in gastric cancer survivors after gastrectomy [15]. In parallel with this result, another study found that cardiovascular risk factors such as triglycerides, low-density lipoprotein cholesterol, and body weight were decreased in gastric cancer patients after gastrectomy [16]. However, precise information on the incidence of CVD in gastric cancer patients within the AYA population remains lacking.
With the expected poor prognosis of AYA gastric cancer patients, finding a way to diminish preventable factors, such as those associated with CVD, is in demand. Therefore, there is a compelling need for targeted research aimed at investigating the specific CVD risk level within this distinct population. The present study sought to investigate the CVD risk among AYAs with gastric cancer compared to non-cancer controls and to calculate the risk of CVD according to treatment modality.
Methods
Data sources
We performed a retrospective, population-based cohort study using the Korean National Health Insurance Service (K-NHIS) database. Korea has a mandatory social insurance system with insurance premiums that are determined by income level and not by health status. The K-NHIS is a single insurer that covers approximately 97% of the population, while the remaining 3% of beneficiaries are covered by the Medical Aid Program. Data on the use of medical facilities and records of prescriptions with International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10), diagnosis codes are gathered by the NHIS. The K-NHIS claims database also includes information on demographics, medical treatment, procedures, prescription drugs, diagnostic codes, and hospital use. Vital status and cause of death were obtained from death certificates collected by Statistics Korea at the Ministry of Strategy and Finance of South Korea [17]. Use of the K-NHIS database was approved by the NHIS review committee.
Definition of AYA cancer survivors
The main exposure was the incidence of cancer in participants 15–39 years old. To define incident cancer, we used a special registration code V193 in addition to the relevant ICD-10 diagnosis code. The K-NHIS has established a special copayment-reduction program to enhance health coverage and reduce the financial burden of patients with cancer. Once cancer patients are registered in the system, they pay only 5% of the total medical bill incurred for cancer-related medical care. Since enrollment in this copayment-reduction program is indicated by a special copayment-reduction code for cancer (V193) and requires a medical certificate from a physician, the cancer diagnoses included in this study are considered to be sufficiently reliable, and this method has been used in previous studies [18].
Study population
For this study, we considered all Korean men and women aged 15–39 years enrolled between 2006 and 2019 in the K-NHIS database between 2005 and 2020. Data access was restricted by a data-share policy; we selected all patients with cancer defined by the presence of ICD-10 code C or a special copayment reduction code for cancer (V193) between 2006 and 2019 (n = 681,752) and fourfold the number of age- and sex-matched samples of men and women who did not develop cancer during the study period (n = 2,670,558). To select newly diagnosed cancer as the exposure and incident cases of CVD as the outcome, we excluded 109,269 participants with CVD (n = 66,317) or any cancer (n = 45,732) before January 1, 2006.
Among the eligible participants (n = 3,243,041), we mimicked sequential emulation of the target trial (detailed methods are presented in the Statistical Analysis section) [19, 20]. The number of cloned participants aged 15–39 years enrolled in the K-NHIS database between 2006 and 2019 was 62,985,785. During the process of enrolling the new cohort, we excluded participants who had any cancer (n = 1,570,072) or a history of CVD at each baseline point (n = 1,859,670). Participants who met the eligibility criteria in the previous cohort were excluded from the next cohort if they were > 40 years old, had cancer or a history of CVD, or died prior to the start date. These processes were repeated every 6 months until June 1, 2019 (n = 59,561,447/unique n = 3,108,601). Since our focus was on AYA gastric cancer, patients who underwent gastric cancer surgery, including endoscopic operation of an upper gastrointestinal tumor and endoscopic submucosal dissection, as well as total and subtotal gastrectomy, within the 2 months prior to diagnosis and within 1 year thereafter with ICD-10 diagnosis code (C16) were selected for the gastric cancer group (n = 6562). Then, we also selected a threefold larger control group using propensity score matching (n = 19,678) (Fig. 1).
The Institutional Review Board of the Samsung Medical Center approved the study and waived the requirement for informed consent because K-NHIS data were de-identified (SMC 2022-03-028).
Study outcomes
The primary endpoint is a composite outcome of any cardiac outcomes, including myocardial infarction (ICD-10: I21–I22), stroke (ICD-10: I60–I64), heart failure (ICD-10: I50), cerebrovascular disease (ICD-10: I63–I69), atrial fibrillation (ICD-10: I48), arrhythmia (ICD-10: I47–I49), cardiomyopathy (ICD-10: I42–I43, I23.5), valvular heart disease (ICD-10: I01–I08, I34–I37), venous thromboembolism (VTE): deep venous thromboembolism (DVT) (ICD-10: I80.1–I80.3), and pulmonary embolism (PE) (ICD-10: I26). The cardiovascular outcomes were identified by diagnostic records, according to the ICD-10 codes from either outpatient visits or hospitalization. The definitions of outcomes are summarized in Supplemental Table 1. In regard to myocardial infarction diagnosis, 93% accuracy was achieved in the validation study [21].
Other variables
For the covariates, we included age, sex, comorbidities, income, and residential area at baseline. The presence of diabetes (ICD-10, E100-E149), hypertension (ICD-10, I10-I15), or dyslipidemia (ICD-10, E780-E785) was defined as having had at least one clinic visit or hospitalization with the corresponding ICD-10 code within the previous year. Data on income were obtained from the insurance eligibility database. Income level was categorized by percentile (≤ 30th, > 30th– ≤ 70th, and > 70th percentiles). Residential area was classified as metropolitan or rural. Metropolitan areas were defined as Seoul, six metropolitan cities, and 15 cities with > 5,00,000 residents that have been officially designated as municipal cities (http://www.mois.go.kr). We conducted a sensitivity analysis to examine whether body mass index (BMI), alcohol drinking, smoking, and physical activity have an additional impact on CVD outcome. For this analysis, we restricted the participants to those who underwent a health screening exam 4 years prior to baseline.
Statistical analysis
The participants were then classified into two groups based on whether they developed gastric cancer or not. In this process, we generated a propensity score using logistic regression with incident cancer as the outcome variable and age, sex, income, residential area, and the presence of comorbidities (diabetes mellitus, hypertension, and hyperlipidemia) at cohort entry as covariates. Then, a 1:3 matching ratio was applied using the propensity score through greedy matching methods (caliper < 0.1). To compare the distribution of variables used for matching, a standardized mean difference between the gastric cancer and control groups was estimated. The variables used for matching were updated based on the first date for each subsequent cohort.
The primary endpoint was development of CVD. Each endpoint was analyzed separately, and we included only participants who had not experienced the endpoint of interest prior to the endpoint analysis. We followed the participants from the baseline of each subsequent cohort until the development of CVD, death, or December 2020, whichever occurred first. After completing all processes, we pooled the data from all trials into a single model and included the day at baseline of each cohort.
The cumulative incidence of each outcome was estimated with the Kaplan–Meier method, and log-rank tests were applied to evaluate differences between the groups. We calculated hazard ratios (HR) with 95% confidence intervals (CIs) for clinical outcome incidence using a Cox regression model. The time scale was the calendar year. We examined the proportional hazards assumption using plots of the log (-log) survival function and Schoenfeld residuals.
In sensitivity analysis, to account for competing risks due to mortality, we fitted a proportional subdistribution hazards (subHR) regression model [22] with death as the competing event. Since the year 2020 was complicated by the coronavirus disease 2019 pandemic, we performed a separate sensitivity analysis to exclude the year 2020. To understand the differential impacts of treatment modalities on the outcomes of interest, additional subgroup analyses were performed in the surgery-only group and the chemotherapy group. These groups were compared to their matched controls from the general population without gastric cancer at baseline. In addition, individual control group tests were conducted for each subgroup. We also performed multivariable Cox regression to find risk factors influencing the incidence of CVD among AYA gastric cancer patients after adjusting for covariates of BMI, smoking status, alcohol consumption and regular physical activity. As the risk of VTE significantly increased in AYA gastric cancer patients, we also presented risk factors affecting the incidence of VTE.
All P values were two-sided, and P < 0.05 was considered statistically significant. Analyses were performed with SAS® Visual Analytics (SAS Institute Inc., Cary, NC, USA) and R 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics
The mean age of study participants was 35 years, and 44% of participants were men (Table 1). All standardized mean differences between the control and AYA gastric cancer groups were < 0.1. Most AYA gastric cancer patients received only surgery (64.8%), followed by surgery with chemotherapy (27.3%).
Difference in CVD risk between AYA gastric cancer patients and matched controls
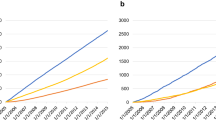
During follow-up (median, 6.5 years), 1535 participants developed CVD (Table 2). The cumulative incidence of CVD was consistently higher in participants with gastric cancer compared to those without gastric cancer during the entire follow-up period (Fig. 2B). The HR for CVD when comparing AYA gastric cancer patients to controls was 1.18 (95% CI 1.05–1.33). However, the association became no significant after competing risk analysis (subHR, 1.00; 95% CI 0.89–1.12). There were no significant differences in BMI, smoking status, drinking status, and physical activity between AYA cancer survivors and controls (Supplemental Table 2). After adjusting these lifestyle covariates, the results were consistent with main findings (Supplemental Table 3).
When stratified by specific outcomes, there was no significant difference in ischemic heart disease, cerebrovascular disease, heart failure, valvular heart disease, arrhythmia, cardiomyopathy, or valvular heart disease between the AYA gastric cancer patients and the control group. The cumulative incidence rates of DVT and PE were consistently higher in AYA gastric cancer patients compared to individuals without gastric cancer during the entire follow-up period (Fig. 2 C, D). The HRs for DVT and PE, when comparing AYA gastric cancer patients to individuals without gastric cancer, were 3.93 (95% CI 3.06–14.67) and 6.58 (95% CI 3.06–14.67), respectively. This association remained significant in competing risk analyses. In the sensitivity analysis using data recorded until 2019, the trends were similar (Supplemental Table 4).
Subgroup analysis by chemotherapy
In the subgroup analysis, patients who received chemotherapy showed a greater risk of CVD (HR, 1.60; 95% CI 1.30–1.96) (Table 3). Compare to the control group, the AYA gastric cancer group receiving chemotherapy also showed a significant increase in stroke (HR, 2.33; 95% CI 1.21–4.26), heart failure (HR, 2.38; 95% CI 2.30–4.34), atrial fibrillation (HR, 2.31; 95% CI 1.11–4.82), DVT (HR, 10.33; 95% CI 6.28–16.99), and PE (HR, 23.71; 95% CI 10.42–53.92), but not myocardial infarction, valvular heart disease, arrhythmia, or cardiomyopathy.
The AYA gastric cancer group without chemotherapy did not show a significant difference in CVD risk compared to the control group (HR, 0.99; 95% CI 0.86–1.14). However, the risk of DVT remained high in the AYA gastric cancer group without chemotherapy compared to the control group (HR, 1.97; 95% CI 1.13–3.41). The results were similar when we selected individual matched group within each subgroup (Supplemental Table 5).
Factors associated with CVD and VTE
Among AYA gastric cancer patients, those with hypertension (HR, 1.58; 95% CI 1.10–2.27) or hyperlipidemia (HR, 1.46; 95% CI 1.06–2.20) had a greater risk of CVD incidence (Table 4). Patients with chemotherapy also had a greater risk of VTE incidence, albeit without statistical significance (HR, 5.11; 95% CI 0.69–38.04).
Discussion
This large population-based cohort study revealed an increased risk of CVD in AYAs with gastric cancer, including a significant increase in both DVT and PE. This increased risk of CVD was more prominent in AYA gastric cancer patients who underwent chemotherapy, while the risk of DVT was high regardless of chemotherapy. In addition, we not only examined the CVD risk of AYA gastric cancer patients but also identified the risk factors associated with an increased CVD risk. To the best of our knowledge, the specific incidence of CVD in AYAs with gastric cancer alone has not been reported previously [11, 12, 23], making this the first study to investigate this incidence. Previous studies have either excluded gastric cancer from investigations into the most common cancers [4] largely due to its lower incidence in Western populations and AYAs or have included it as a gastrointestinal tract cancers.
Contrary to AYA gastric cancer, a study that enrolled older gastric patients reported that gastric cancer was associated with decreased risk of coronary heart disease and ischemic stroke [15]. This may be attributed to the weight loss associated with the reduced stomach capacity after gastrectomy, leading to improvements in metabolic risk factors related to CVD [16, 24]. However, the favorable changes in lipid profile diminished as time passed after surgery [24]. From this result, we can infer that the immediate favorable effect of gastrectomy on metabolic risk factors could be diminished in AYA gastric cancer patients compared to older patients as time passes over a longer period of survivorship. In addition, since the prevalence of known CVD risk factors such as obesity [25] and metabolic syndrome [26] is high among older patients compared to young individuals, the positive outcome from gastrectomy may only be prominent among older gastric cancer patients and not AYA gastric cancer patients.
AYA cancer patients are not eligible for national cancer screening programs. In Korea, the biannual health check-up, which includes upper endoscopy, is recommended for individuals aged ≥ 40 years [15]. Therefore, unless there are specific symptoms, it is not easy to detect gastric cancer at an early stage among young individuals. As a result, AYA gastric cancer cases often present in an advanced stage [3, 27]. In addition, the histological types of cancer observed in AYA gastric cancer patients are often poorly differentiated or of the diffuse type [3, 4, 27], and these cases are known to be more aggressive compared to those in adults, leading to a reported poor prognosis. The location of gastric cancer is also frequently observed in the middle third or entirety of the stomach [4]. Another characteristic of AYA gastric cancer is a female predominance [3, 4, 27], which was consistently observed in our study.
In our study, the risk of overall CVD in AYA gastric cancer patients who received chemotherapy was 1.6 times that of the matched controls. Since younger gastric cancer patients are diagnosed relatively late and show more aggressive biological cancer behaviors, as previously mentioned, they are more likely to undergo additional chemotherapy rather than surgery alone. Previous population-based studies have reported that gastric cancer patients have a higher CVD mortality rate than that of non-cancer individuals, with a relative risk ranging from 1.13–3.93 [5, 6, 28]. In particular, younger age at cancer diagnosis is associated with increased CVD mortality [6], which can be explained by cumulative cardiac damage due to prolonged exposure to chemotherapy [10, 13] or radiotherapy [28]. Consequently, it is speculated that younger age at chemotherapy initiation leads to longer exposure to chemotherapy, contributing to more frequent development of CVD.
Notably, in this study, we demonstrated a significantly increased risk for VTE in AYA gastric cancer patients. The risk of PE was increased 15.10-fold and that of DVT was increased 6.25-fold in those who underwent surgery and chemotherapy. Moreover, a sustained increased risk for DVT and PE was observed in AYA gastric cancer patients throughout the study follow-up period.
Our results are concurrent with those of previous studies reporting an increased VTE risk among gastric cancer patients [29, 30]. Gastric cancer is reported to be one of the common solid tumors with the highest risk of VTE [31], and gastric cancer patients have been reported to experience more cancer-associated thrombotic events [32]. Cancer initiates thrombotic events by producing tissue factors and cancer procoagulants, which lead to amplification of the coagulation cascade [33]. Therefore, an increased risk of VTE was speculated in AYA gastric cancer survivors compared to a non-cancer control group due to increased platelet activation and the formation of thrombi. An increase in venous stasis caused by the surgical procedure itself or postoperative performance status and immobility could be risk factor for VTE as well [34], accounting for the 2.08-fold increased risk of DVT in AYA gastric cancer patients who only received surgery in our study. Most of all, previous studies demonstrated an increased risk of VTE in patients with advanced and metastatic gastric cancer, who are more likely to undergo chemotherapy [35,36,37]. A study conducted in Korea investigated the incidence of VTE in advanced gastric cancer patients and found that patients with inoperable gastric cancer, who were most likely to receive anticancer treatment, had a high risk of developing VTE [38], with a 1-year cumulative incidence rate of VTE of 3.5% documented in advanced inoperable gastric cancer patients. Meanwhile, another study of Japanese gastric cancer patients reported an incidence of VTE of 18% in metastatic stomach cancer patients receiving chemotherapy [39]. Here, we observed the risk of VTE in AYA gastric cancer to be higher than what has been reported in other studies. This could be attributed to the fact that AYA gastric cancer patients undergo prolonged exposure to chemotherapy, and a young-aged non-cancer control group is less likely to experience VTE events.
Our study revealed a significant increase in the risk of CVD among AYA gastric cancer survivors, especially those who received chemotherapy and had comorbidities such as hypertension and dyslipidemia. Therefore, AYA gastric cancer survivors who receive chemotherapy and have comorbidities require more medical attention during surveillance periods.
Our study also confirms a meaningful increase in the risk of VTE in AYA gastric cancer survivors regardless of chemotherapy. It is suggested that VTE is associated with decreased life expectancy in cancer patients [40], so recognizing its risk and preventing it adequately by providing thromboprophylaxis to AYA gastric cancer survivors would relieve additional complications and burdens. Moreover, further investigation is warranted to elucidate the underlying mechanism of increased VTE among AYA gastric cancer survivors.
Study limitations
Despite the strength of enrolling a large, unselected Korean population, this study has several limitations. First, due to the use of administrative data, we lacked detailed clinicopathological information, such as cancer stage and histology. However, we could infer such details based on the information about the treatments received by the patients. Second, the use of claims data can involve inaccurate outcome definitions, so the actual incidence could be either underrated or exaggerated. Finally, this study focused on Korean individuals, whose AYA cancer incidence may differ from that of Western populations, so generalizing the results might be challenging.
Conclusion
In conclusion, this large population-based cohort study confirmed a potential risk for CVD, especially VTE, in AYA gastric cancer survivors. This risk was prominently high among patients who had received chemotherapy. Our results highlight the importance of closely monitoring AYA gastric cancer survivors to enhance their prognosis by proactively mitigating the incidence of CVD, especially including preventing VTE events by providing prompt prophylactic treatment. Further research is warranted to elucidate the underlying mechanisms contributing to this increased risk.
References
Thrift AP, El-Serag HB. Burden of gastric cancer. Clin Gastroenterol Hepatol. 2020;18(3):534–42. https://doi.org/10.1016/j.cgh.2019.07.045.
Arnold M, Park JY, Camargo MC, Lunet N, Forman D, Soerjomataram I. Is gastric cancer becoming a rare disease? a global assessment of predicted incidence trends to 2035. Gut. 2020;69(5):823–9. https://doi.org/10.1136/gutjnl-2019-320234.
Ławniczak M, Gawin A, Jaroszewicz-Heigelmann H, Rogoza-Mateja W, Białek A, Kulig J, et al. Analysis of clinicopathologic characteristics of gastric cancer in patients ≤40 and ≥40 years of age. Scand J Gastroenterol. 2020;55(1):62–6. https://doi.org/10.1080/00365521.2019.1699597. (Epub 2019/12/20 PubMed PMID: 31852320).
Isobe T, Hashimoto K, Kizaki J, Miyagi M, Aoyagi K, Koufuji K, et al. Characteristics and prognosis of gastric cancer in young patients. Oncol Rep. 2013;30(1):43–9. https://doi.org/10.3892/or.2013.2467. (Epub 2013/05/16 PubMed PMID: 23674196).
Florido R, Daya NR, Ndumele CE, Koton S, Russell SD, Prizment A, et al. Cardiovascular disease risk among cancer survivors: the atherosclerosis risk in communities (ARIC) study. J Am Coll Cardiol. 2022;80(1):22–32. https://doi.org/10.1016/j.jacc.2022.04.042. (Epub 2022/07/01 PubMed PMID: 35772913).
Sturgeon KM, Deng L, Bluethmann SM, Zhou S, Trifiletti DM, Jiang C, et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J. 2019;40(48):3889–97. https://doi.org/10.1093/eurheartj/ehz766. (Epub 2019/11/26 PubMed PMID: 31761945).
Yang L, Zhang N, Yue Q, Song W, Zheng Y, Huang S, et al. Long-term atherosclerotic cardiovascular disease risk in patients with cancer: a population-based study. Curr Probl Cardiol. 2023;48(7):101693. https://doi.org/10.1016/j.cpcardiol.2023.101693. (Epub 2023/03/17 PubMed PMID: 36924906).
Strongman H, Gadd S, Matthews A, Mansfield KE, Stanway S, Lyon AR, et al. Medium and long-term risks of specific cardiovascular diseases in survivors of 20 adult cancers: a population-based cohort study using multiple linked UK electronic health records databases. Lancet. 2019;394(10203):1041–54. https://doi.org/10.1016/S0140-6736(19)31674-5.
Berkman AM, Andersen CR, Roth ME, Gilchrist SC. Cardiovascular disease in adolescent and young adult cancer survivors: impact of sociodemographic and modifiable risk factors. Cancer. 2023;129(3):450–60. https://doi.org/10.1002/cncr.34505. (Epub 2022/12/06 PubMed PMID: 36464957).
Henson KE, Reulen RC, Winter DL, Bright CJ, Fidler MM, Frobisher C, et al. Cardiac mortality among 200 000 five-year survivors of cancer diagnosed at 15 to 39 years of age: the teenage and young adult cancer survivor study. Circulation. 2016;134(20):1519–31. https://doi.org/10.1161/circulationaha.116.022514. (Epub 2016/11/09 PubMed PMID: 27821538).
Rugbjerg K, Mellemkjaer L, Boice JD, Køber L, Ewertz M, Olsen JH. Cardiovascular disease in survivors of adolescent and young adult cancer: a Danish cohort study 1943–2009. J Natl Cancer Inst. 2014;106(6):dju110. https://doi.org/10.1093/jnci/dju110. (Epub 2014/05/23 PubMed PMID: 24848622).
Keegan THM, Kushi LH, Li Q, Brunson A, Chawla X, Chew HK, et al. Cardiovascular disease incidence in adolescent and young adult cancer survivors: a retrospective cohort study. J Cancer Surviv. 2018;12(3):388–97. https://doi.org/10.1007/s11764-018-0678-8. (Epub 2018/02/11 PubMed PMID: 29427203).
Wang L, Wang F, Chen L, Geng Y, Yu S, Chen Z. Long-term cardiovascular disease mortality among 160 834 5-year survivors of adolescent and young adult cancer: an American population-based cohort study. Eur Heart J. 2021;42(1):101–9. https://doi.org/10.1093/eurheartj/ehaa779. (Epub 2020/11/07 PubMed PMID: 33156911).
Nichols HB, Baggett CD, Engel SM, Getahun D, Anderson C, Cannizzaro NT, et al. The adolescent and young adult (AYA) horizon study: an AYA cancer survivorship cohort. Cancer Epidemiol Biomark Prev. 2021;30(5):857–66. https://doi.org/10.1158/1055-9965.Epi-20-1315. (Epub 2021/02/24 PubMed PMID: 33619021).
Shin DW, Suh B, Park Y, Lim H, Suh YS, Yun JM, et al. Risk of Coronary heart disease and ischemic stroke incidence in gastric cancer survivors: a nationwide study in Korea. Ann Surg Oncol. 2018;25(11):3248–56. https://doi.org/10.1245/s10434-018-6635-y. (Epub 2018/07/26 PubMed PMID: 30043317).
Ha TK, Seo YK, Kang BK, Shin J, Ha E. Cardiovascular risk factors in gastric cancer patients decrease 1 year after gastrectomy. Obes Surg. 2016;26(10):2340–7. https://doi.org/10.1007/s11695-016-2085-4. (Epub 2016/02/11 PubMed PMID: 26861008).
Lee J, Lee JS, Park SH, Shin SA, Kim K. Cohort profile: the national health insurance service-national sample cohort (NHIS-NSC), South Korea. Int J Epidemiol. 2017;46(2):e15. https://doi.org/10.1093/ije/dyv319. (Epub 2016/01/30 PubMed PMID: 26822938).
Yang MS, Park M, Back JH, Lee GH, Shin JH, Kim K, et al. Validation of cancer diagnosis based on the national health insurance service database versus the national cancer registry database in Korea. Cancer Res Treat. 2022;54(2):352–61. https://doi.org/10.4143/crt.2021.044. (Epub 2021/08/07 PubMed PMID: 34353000).
García-Albéniz X, Hsu J, Bretthauer M, Hernán MA. Effectiveness of screening colonoscopy to prevent colorectal cancer among medicare beneficiaries aged 70 to 79 years: a prospective observational study. Ann Intern Med. 2017;166(1):18–26. https://doi.org/10.7326/m16-0758. (Epub 20160927 PubMed PMID: 27669524).
Hernán MA, Sauer BC, Hernández-Díaz S, Platt R, Shrier I. Specifying a target trial prevents immortal time bias and other self-inflicted injuries in observational analyses. J Clin Epidemiol. 2016;79:70–5. https://doi.org/10.1016/j.jclinepi.2016.04.014. (Epub 2016/05/31 PubMed PMID: 27237061).
Kim H, Yun JE, Lee SH, Jang Y, Jee SH. Validity of the diagnosis of acute myocardial infarction in Korean national medical health insurance claims data: the Korean Heart Study. Korean Circ J. 2012;42(1):10–5. https://doi.org/10.4070/kcj.2012.42.1.10.
Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. https://doi.org/10.1080/01621459.1999.10474144.
Chao C, Xu L, Bhatia S, Cooper R, Brar S, Wong FL, et al. Cardiovascular disease risk profiles in survivors of adolescent and young adult (AYA) cancer: the kaiser permanente aya cancer survivors study. J Clin Oncol. 2016;34(14):1626–33. https://doi.org/10.1200/jco.2015.65.5845. (Epub 2016/03/10 PubMed PMID: 26951318).
Lee JW, Kim EY, Yoo HM, Park CH, Song KY. Changes of lipid profiles after radical gastrectomy in patients with gastric cancer. Lipids Health Dis. 2015;14(1):21. https://doi.org/10.1186/s12944-015-0018-1.
Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007–2008 to 2015–2016. JAMA. 2018;319(16):1723–5. https://doi.org/10.1001/jama.2018.3060.
Huh JH, Kang DR, Kim JY, Koh KK. Metabolic syndrome fact sheet 2021: executive report. Cardiometab Syndr J. 2021;1(2):125–34.
Park HJ, Ahn JY, Jung HY, Lim H, Lee JH, Choi KS, et al. Clinical characteristics and outcomes for gastric cancer patients aged 18–30 years. Gastric Cancer. 2014;17(4):649–60. https://doi.org/10.1007/s10120-013-0331-1. (Epub 2014/01/15 PubMed PMID: 24414087).
Liu E, Guan X, Wei R, Jiang Z, Liu Z, Wang G, et al. Association between radiotherapy and death from cardiovascular disease among patients with cancer: a large population-based cohort study. J Am Heart Assoc. 2022;11(6):e023802. https://doi.org/10.1161/jaha.121.023802. (Epub 2022/03/08 PubMed PMID: 35253473).
Jung YJ, Seo HS, Park CH, Jeon HM, Kim J-I, Yim HW, et al. Venous thromboembolism incidence and prophylaxis use after gastrectomy among Korean patients with gastric adenocarcinoma: the PROTECTOR randomized clinical trial. JAMA Surg. 2018;153(10):939–46. https://doi.org/10.1001/jamasurg.2018.2081.
Khorana AA, Dalal M, Lin J, Connolly GC. Incidence and predictors of venous thromboembolism (VTE) among ambulatory high-risk cancer patients undergoing chemotherapy in the United States. Cancer. 2013;119(3):648–55. https://doi.org/10.1002/cncr.27772.
Moik F, Ay C, Pabinger I. Risk prediction for cancer-associated thrombosis in ambulatory patients with cancer: past, present and future. Thromb Res. 2020;191(Suppl 1):S3-s11. https://doi.org/10.1016/s0049-3848(20)30389-3. (Epub 2020/08/02 PubMed PMID: 32736775).
Khorana AA, Mackman N, Falanga A, Pabinger I, Noble S, Ageno W, et al. Cancer-associated venous thromboembolism. Nat Rev Dis Primers. 2022;8(1):11. https://doi.org/10.1038/s41572-022-00336-y.
Mukai M, Oka T. Mechanism and management of cancer-associated thrombosis. J Cardiol. 2018;72(2):89–93. https://doi.org/10.1016/j.jjcc.2018.02.011.
Osaki T, Saito H, Fukumoto Y, Kono Y, Murakami Y, Shishido Y, et al. Risk and incidence of perioperative deep vein thrombosis in patients undergoing gastric cancer surgery. Surg Today. 2018;48(5):525–33. https://doi.org/10.1007/s00595-017-1617-4. (Epub 2017/12/14 PubMed PMID: 29234961).
Kimura Y, Oki E, Ando K, Saeki H, Kusumoto T, Maehara Y. Incidence of venous thromboembolism following laparoscopic surgery for gastrointestinal cancer: a single-center, prospective cohort study. World J Surg. 2016;40(2):309–14. https://doi.org/10.1007/s00268-015-3234-y.
Wada T, Fujiwara H, Morita S, Fukagawa T, Katai H. Incidence of and risk factors for preoperative deep venous thrombosis in patients undergoing gastric cancer surgery. Gastric Cancer. 2017;20(5):872–7. https://doi.org/10.1007/s10120-017-0690-0. (Epub 2017/01/26 PubMed PMID: 28120128).
Horsted F, West J, Grainge MJ. Risk of venous thromboembolism in patients with cancer: a systematic review and meta-analysis. PLoS Med. 2012;9(7):e1001275. https://doi.org/10.1371/journal.pmed.1001275. (Epub 2012/08/04 PubMed PMID: 22859911).
Kang MJ, Ryoo BY, Ryu MH, Koo DH, Chang HM, Lee JL, et al. Venous thromboembolism (VTE) in patients with advanced gastric cancer: an Asian experience. Eur J Cancer. 2012;48(4):492–500. https://doi.org/10.1016/j.ejca.2011.11.016. (Epub 2011/12/16 PubMed PMID: 22169121).
Takayoshi K, Kusaba H, Aikawa T, Koreishi S, Sagara K, Nakano M, et al. Hypoalbuminemia for the prediction of venous thromboembolism and treatment of direct oral anticoagulants in metastatic gastric cancer patients. Gastric Cancer. 2019;22(5):988–98. https://doi.org/10.1007/s10120-019-00930-2. (Epub 2019/02/23 PubMed PMID: 30788749).
Khorana AA. Venous thromboembolism and prognosis in cancer. Thromb Res. 2010;125(6):490–3. https://doi.org/10.1016/j.thromres.2009.12.023. (Epub 2010/01/26 PubMed PMID: 20097409).
Acknowledgements
None.
Funding
Open Access funding enabled and organized by Seoul National University. This work was supported by the Institution of Quality of Life in Cancer funded by Samsung Fire & Marine Insurance and the National Research Foundation of Korea Grant funded by the Korean Government (NRF‑2022R1A2C1013119). Funding sources did not involve study design, analysis and interpretation of data, writing of the report and the decision to submit the article for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Choi, H.L., Kang, D., Kim, H. et al. Increased cardiovascular disease risk among adolescents and young adults with gastric cancer. Gastric Cancer (2024). https://doi.org/10.1007/s10120-024-01540-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10120-024-01540-3