Abstract
Background
Laparoscopy-assisted gastrectomy (LG) is rapidly gaining popularity owing to its minimal invasiveness. Previous studies have found that compared with two-dimensional (2D)-LG, three-dimensional (3D)-LG showed better short-term outcomes. However, the long-term oncological outcomes in patients with locally resectable gastric cancer (GC) remain controversial.
Methods
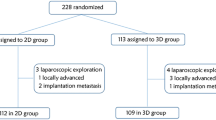
In this noninferiority, open-label, randomized clinical trial, a total of 438 eligible GC participants were randomly assigned in a 1:1 ratio to either 3D-LG or 2D-LG from January 2015 to April 2016. The primary endpoint was operating time, while the secondary endpoints included 5-year overall survival (OS), disease-free survival (DFS), and recurrence pattern.
Results
Data from 401 participants were included in the per-protocol analysis, with 204 patients in the 3D group and 197 patients in the 2D group. The 5-year OS and DFS rates were comparable between the 3D and 2D groups (5-year OS: 70.6% vs. 71.1%, Log-rank P = 0.743; 5-year DFS: 68.1% vs. 69.0%, log-rank P = 0.712). No significant differences were observed between the 3D and 2D groups in the 5-year recurrence rate (28.9% vs. 28.9%, P = 0.958) or recurrence time (mean time, 22.6 vs. 20.5 months, P = 0.412). Further stratified analysis based on the type of gastrectomy, postoperative pathological staging, and preoperative BMI showed that the 5-year OS, DFS, and recurrence rates of the 3D group in each subgroup were similar to those of the 2D group (all P > 0.05).
Conclusions
For patients with locally resectable GC, 3D-LG performed by experienced surgeons in high-volume professional institutions can achieve long-term oncological outcomes comparable to those of 2D-LG.
Registration number
NCT02327481 (http://clinicaltrials.gov).
Graphical abstract



Similar content being viewed by others
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet (London). 2020;396(10251):635–48.
Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4(2):146–8.
Iino I, Sakaguchi T, Kikuchi H, Miyazaki S, Fujita T, Hiramatsu Y, Ohta M, Kamiya K, Ushio T, Takehara Y, et al. Usefulness of three-dimensional angiographic analysis of perigastric vessels before laparoscopic gastrectomy. Gastric Cancer Off J Int Gastric Cancer Assoc Jpn Gastric Cancer Assoc. 2013;16(3):355–61.
Smith R, Schwab K, Day A, Rockall T, Ballard K, Bailey M, Jourdan I. Effect of passive polarizing three-dimensional displays on surgical performance for experienced laparoscopic surgeons. Br J Surg. 2014;101(11):1453–9.
Sørensen SMD, Savran MM, Konge L, Bjerrum F. Three-dimensional versus two-dimensional vision in laparoscopy: a systematic review. Surg Endosc. 2016;30(1):11–23.
Park CH, Park JC, Chung H, Shin SK, Lee SK, Cheong J-H, Hyung WJ, Lee YC, Noh SH, Kim CB. Impact of the surveillance interval on the survival of patients who undergo curative surgery for gastric cancer. Ann Surg Oncol. 2016;23(2):539–45.
Au KP, Chan MY, Chu KW, Kwan CLY, Ma KW, She WH, Tsang SHY, Dai WC, Cheung TT, Chan ACY. Impact of three-dimensional (3D) visualization on laparoscopic hepatectomy for hepatocellular carcinoma. Ann Surg Oncol. 2022;29(11):6731–44.
Agrusa A, di Buono G, Chianetta D, Sorce V, Citarrella R, Galia M, Vernuccio L, Romano G, Gulotta G. Three-dimensional (3D) versus two-dimensional (2D) laparoscopic adrenalectomy: a case–control study. Int J Surg (London). 2016;28(Suppl 1):S114–7.
Ashraf A, Collins D, Whelan M, O’Sullivan R, Balfe P. Three-dimensional (3D) simulation versus two-dimensional (2D) enhances surgical skills acquisition in standardised laparoscopic tasks: a before and after study. Int J Surg (Lond). 2015;14:12–6.
Gabrielli ME, Saun TJ, Jung JJ, Grantcharov TP. Assessment of 3-dimensional vs. 2-dimensional imaging and technical performance using a multiport intraoperative data capture and analytic system for patients undergoing laparoscopic roux-en-y gastric bypass surgery. JAMA Netw Open. 2020;3(1):e1920084.
Katai H, Mizusawa J, Katayama H, Takagi M, Yoshikawa T, Fukagawa T, Terashima M, Misawa K, Teshima S, Koeda K, et al. Short-term surgical outcomes from a phase III study of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric cancer: Japan Clinical Oncology Group Study JCOG0912. Gastric Cancer Off J Int Gastric Cancer Assoc Jpn Gastric Cancer Assoc. 2017;20(4):699–708.
Zheng C-H, Lu J, Zheng H-L, Li P, Xie J-W, Wang J-B, Lin J-X, Chen Q-Y, Cao L-L, Lin M, et al. Comparison of 3D laparoscopic gastrectomy with a 2D procedure for gastric cancer: a phase 3 randomized controlled trial. Surgery. 2018;163(2):300–4.
Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17(12):3077–9.
Gong J-Q, Cao Y-K, Wang Y-H, Zhang G-H, Wang P-H, Luo G-D. Learning curve for hand-assisted laparoscopic D2 radical gastrectomy. World J Gastroenterol. 2015;21(5):1606–13.
Morris JS. Disease-free survival is a promising surrogate for overall survival in colorectal cancer studies. J Natl Cancer Inst. 2022;114(1):5–6.
Hung JJ, Hsu WH, Hsieh CC, Huang BS, Huang MH, Liu JS, Wu YC. Post-recurrence survival in completely resected stage I non-small cell lung cancer with local recurrence. Thorax. 2009;64(3):192–6.
Ajao MO, Larsen CR, Manoucheri E, Goggins ER, Rask MT, Cox MKB, Mushinski A, Gu X, Cohen SL, Rudnicki M, et al. Two-dimensional (2D) versus three-dimensional (3D) laparoscopy for vaginal cuff closure by surgeons-in-training: a randomized controlled trial. Surg Endosc. 2020;34(3):1237–43.
Mashiach R, Mezhybovsky V, Nevler A, Gutman M, Ziv A, Khaikin M. Three-dimensional imaging improves surgical skill performance in a laparoscopic test model for both experienced and novice laparoscopic surgeons. Surg Endosc. 2014;28(12):3489–93.
Chen Q-Y, Xie J-W, Zhong Q, Wang J-B, Lin J-X, Lu J, Cao L-L, Lin M, Tu R-H, Huang Z-N, et al. Safety and efficacy of indocyanine green tracer-guided lymph node dissection during laparoscopic radical gastrectomy in patients with gastric cancer: a randomized clinical trial. JAMA Surg. 2020;155(4):300–11.
Wang J, Wang Q, Dong J, Yang K, Ji S, Fan Y, Wang C, Ma Q, Wei Q, Ji G. Total laparoscopic uncut roux-en-Y for radical distal gastrectomy: an interim analysis of a randomized, controlled. Clin Trial Ann Surg Oncol. 2021;28(1):90–6.
Hu Y, Huang C, Sun Y, Su X, Cao H, Hu J, Xue Y, Suo J, Tao K, He X, et al. Morbidity and mortality of laparoscopic versus open D2 distal gastrectomy for advanced gastric cancer: a randomized controlled trial. J Clin Oncol Off J Am Soc Clin Oncol. 2016;34(12):1350–7.
Herron DM, Lantis JC, Maykel J, Basu C, Schwaitzberg SD. The 3-D monitor and head-mounted display. A quantitative evaluation of advanced laparoscopic viewing technologies. Surg Endosc. 1999;13(8):751–5.
Wang W-J, Li H-T, Yu J-P, Su L, Guo C-A, Chen P, Yan L, Li K, Ma Y-W, Wang L, et al. Severity and incidence of complications assessed by the Clavien–Dindo classification following robotic and laparoscopic gastrectomy for advanced gastric cancer: a retrospective and propensity score-matched study. Surg Endosc. 2019;33(10):3341–54.
Tokunaga M, Sugisawa N, Kondo J, Tanizawa Y, Bando E, Kawamura T, Terashima M. Early phase II study of robot-assisted distal gastrectomy with nodal dissection for clinical stage IA gastric cancer. Gastric Cancer Off J Int Gastric Cancer Assoc Jpn Gastric Cancer Assoc. 2014;17(3):542–7.
Yang K, Cho M, Roh CK, Seo WJ, Choi S, Son T, Kim H-I, Hyung WJ. Robotic spleen-preserving splenic hilar lymph node dissection during total gastrectomy for gastric cancer. Surg Endosc. 2019;33(7):2357–63.
Jiang Y, Zhao Y, Qian F, Shi Y, Hao Y, Chen J, Li P, Yu P. The long-term clinical outcomes of robotic gastrectomy for gastric cancer: a large-scale single institutional retrospective study. Am J Transl Res. 2018;10(10):3233–42.
Hyung WJ, Yang HK, Park YK, Lee HJ, An JY, Kim W, Kim HI, Kim HH, Ryu SW, Hur H, Kim MC, Kong SH, Cho GS, Kim JJ, Park DJ, Ryu KW, Kim YW, Kim JW, Lee JH, Han SU. Korean laparoendoscopic gastrointestinal surgery study group. Long-term outcomes of laparoscopic distal gastrectomy for locally advanced gastric cancer: The KLASS-02-RCT Randomized Clinical Trial. J Clin Oncol. 2020;38(28):3304–13.
Kim HH, Han SU, Kim MC, Kim W, Lee HJ, Ryu SW, Cho GS, Kim CY, Yang HK, Park DJ, Song KY, Lee SI, Ryu SY, Lee JH, Hyung WJ. Korean laparoendoscopic gastrointestinal surgery study (KLASS) group effect of laparoscopic distal gastrectomy vs. Open distal gastrectomy on long-term survival among patients with stage I gastric cancer: the KLASS-01 randomized clinical trial. JAMA Oncol. 2019;5(4):506–13.
Lee K, Youn SI, Won Y, Min SH, Park YS, Ahn SH, Park DJ, Kim HH. Prospective randomized controlled study for comparison of 2-dimensional versus 3-dimensional laparoscopic distal gastrectomy for gastric adenocarcinoma. Surg Endosc. 2021;35(2):934–40.
Greenhill C. Gastric cancer. Metformin improves survival and recurrence rate in patients with diabetes and gastric cancer. Nat Rev Gastroenterol Hepatol. 2015;12(3):124.
Chang JS, Kim KH, Yoon HI, Hyung WJ, Rha SY, Kim HS, Lee YC, Lim JS, Noh SH, Koom WS. Locoregional relapse after gastrectomy with D2 lymphadenectomy for gastric cancer. Br J Surg. 2017;104(7):877–84.
Lee JH, Chang KK, Yoon C, Tang LH, Strong VE, Yoon SS. Lauren histologic type is the most important factor associated with pattern of recurrence following resection of gastric adenocarcinoma. Ann Surg. 2018;267(1):105–13.
Lee D, Son S-Y, Kim Y-B, Han S-U, Hur H. Neural invasion is a significant contributor to peritoneal recurrence in signet ring cell gastric carcinoma. Ann Surg Oncol. 2018;25(5):1167–75.
Takahashi Y, Takeuchi T, Sakamoto J, Touge T, Mai M, Ohkura H, Kodaira S, Okajima K, Nakazato H. The usefulness of CEA and/or CA19-9 in monitoring for recurrence in gastric cancer patients: a prospective clinical study. Gastric Cancer Off J Int Gastric Cancer Assoc Jpn Gastric Cancer Assoc. 2003;6(3):142–5.
Kim SH, Song BI, Kim HW, Won KS, Son YG, Ryu SW. Prognostic value of restaging F-18 fluorodeoxyglucose positron emission tomography/computed tomography to predict 3-year post-recurrence survival in patients with recurrent gastric cancer after curative resection. Korean J Radiol. 2020;21(7):829–37.
Cardoso R, Coburn NG, Seevaratnam R, Mahar A, Helyer L, Law C, Singh S. A systematic review of patient surveillance after curative gastrectomy for gastric cancer: a brief review. Gastric Cancer Off J Int Gastric Cancer Assoc Jpn Gastric Cancer Assoc. 2012;15(Suppl 1):S164–7.
Özgüner O, Shkurti T, Huang S, Hao R, Jackson RC, Newman WS, Çavuşoğlu MC. Camera-robot calibration for the da Vinci® robotic surgery system. IEEE Trans Autom Sci Eng. 2020;17(4):2154–61.
Hashizume M, Sugimachi K. Robot-assisted gastric surgery. Surg Clin North Am. 2003;83(6):1429–44.
Tian Y, Guo H, Hu Y, Yang P, Liu Y, Zhang Z, Ding P, Zheng T, Fan L, Zhang Z, et al. Safety and efficacy of robotic-assisted versus laparoscopic distal gastrectomy after neoadjuvant chemotherapy for advanced gastric cancer. Surg Endosc. 2023;1:1.
Shim JH, Kim JG, Jeon HM, Park CH, Song KY. The robotic third arm as a competent analog of an assisting surgeon in radical gastrectomy: impact on short-term clinical outcomes. J Laparoendosc Adv Surg Tech A. 2013;23(5):447–51.
Han D-S, Suh Y-S, Ahn HS, Kong S-H, Lee H-J, Kim W-H, Yang H-K. Comparison of surgical outcomes of robot-assisted and laparoscopy-assisted pylorus-preserving gastrectomy for gastric cancer: a propensity score matching analysis. Ann Surg Oncol. 2015;22(7):2323–8.
Yoon HM, Kim Y-W, Lee JH, Ryu KW, Eom BW, Park JY, Choi IJ, Kim CG, Lee JY, Cho SJ, et al. Robot-assisted total gastrectomy is comparable with laparoscopically assisted total gastrectomy for early gastric cancer. Surg Endosc. 2012;26(5):1377–81.
Lu J, Wu D, Huang JB, Lin J, Xu BB, Xue Z, Zheng HL, Lin GS, Shen LL, Li P, Wang JB, Lin JX, Chen QY, Cao LL, Xie JW, Zheng CH, Huang CM. Comparison of robotic versus laparoscopic versus open distal gastrectomy for locally advanced gastric cancer: a prospective trial-based economic evaluation. Surg Endosc. 2023;37(10):7472–85.
Lu J, Zheng HL, Li P, Xie JW, Wang JB, Lin JX, Chen QY, Cao LL, Lin M, Tu RH, Huang ZN, Huang CM, Zheng CH. A propensity score-matched comparison of robotic versus laparoscopic gastrectomy for gastric cancer: oncological, cost, and surgical stress analysis. J Gastrointest Surg. 2018;22(7):1152–62.
Tian Y, Lin Y, Sun C, Lowe S, Bentley R, Yang P, Guo H, Ding P, Zhang Z, Wang D, et al. Comparison of short-term efficacy and safety between total robotic and total 3D laparoscopic distal radical gastrectomy for gastric cancer in Enhanced Recovery After Surgery (ERAS) protocol: a propensity score matching study. J Robot Surg. 2023;17(3):1151–8.
Fanfani F, Rossitto C, Restaino S, Ercoli A, Chiantera V, Monterossi G, Barbati G, Scambia G. How technology can impact surgeon performance: a randomized trial comparing 3-dimensional versus 2-dimensional laparoscopy in gynecology oncology. J Minim Invasive Gynecol. 2016;23(5):810–7.
Ko JK, Li RH, Cheung VY. Two-dimensional versus three-dimensional laparoscopy: evaluation of physicians’ performance and preference using a pelvic trainer. J Minim Invasive Gynecol. 2015;22(3):421–7.
Acknowledgements
We thank those who have devoted a lot to this study, including nurses, pathologists, further-study surgeons, statisticians, reviewers and editors. Thanks for Dr. Zhi-Hong Huang, Public Technology Service Center, Fujian Medical University. We would like to thank Editage (www.editage.cn) for English language editing. They were not financially compensated for their contributions.
Funding
This study was supported by Province Medical “Creating high-level hospitals, high-level medical centers and key specialty projects” [MWYZ (2021) No. 76], Talent Initiation Fund Project of Fujian Medical University Union Hospital (2022XH041), Yunnan Provincial Science and Technology Department (202105AF150040).
Author information
Authors and Affiliations
Contributions
Concept and design: ZQ, CJY, SGZX, HCM, and ZCH. Acquisition, analysis, or interpretation of data: CJY, SGZX, LGT, and LZY. Drafting of the manuscript: ZQ, CJY, SGZX, LJ, HCM, and ZCH. Statistical analysis: ZQ, CJY, SGZX, and LJ. Administrative, technical, or material support: LZY, LGT, WD, JYM, WJB, LJX, CQY, LJL, XJW, and LP. Supervision: ZQ, CJY, and SGZX.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted with the approval of the Biomedical ethical review committee of Fujian Medical University of China (IRB:2014019).
Consent for publication
This manuscript has not been published or presented elsewhere in part or in entirety and is not under consideration by another journal.
Conflict of interest
All authors have no conflict of interest and no potential benefits. The institutional review boards of all the participating institutions approved the study. The authors have no other disclosures to report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file 1
. Supplemental Digital Content 1. FUGES-001 Study Protocol ver.1.1. (DOCX 15272 kb)
Supplementary file 2
. Supplemental Digital Content 2. eTables and eFigures. eTable 1. Eligibility Criteria for Enrolling Patients. eTable 2. Univariable and Multivariable Cox Regression Analyses of Risk Factors for PRS. eTable 3. Frequencies of Causes of First Recurrence Within 5 Years After Surgery between 3D and 2D Groups in patients with total gastrectomy. eTable 4. Frequencies of Causes of First Recurrence Within 5 Years After Surgery between 3D and 2D Groups in patients with distal gastrectomy. eTable 5. Frequencies of Causes of First Recurrence Within 5 Years After Surgery. between 3D and 2D Groups in patients of pI staging. eTable 6. Frequencies of Causes of First Recurrence Within 5 Years After Surgery between 3D and 2D Groups in patients of pII staging. eTable 7. Frequencies of Causes of First Recurrence Within 5 Years After Surgery between 3D and 2D Groups in patients of pIII staging. eTable 8. Frequencies of Causes of First Recurrence Within 5 Years After Surgery between 3D and 2D Groups in patients of BMI ≤ 25. eTable 9. Frequencies of Causes of First Recurrence Within 5 Years After Surgery between 3D and 2D Groups in patients of BMI > 25. eFig. 1: Cumulative Incidence of Any Recurrence for 3D versus 2D Group within 5 Years after Surgery. eFig. 2: Recurrent site columnar distribution map. eFig. 3: Postoperative recurrence time nuclear density map. eFig. 4: Post-recurrence survival for 3D-Laparoscopic Gastrectomy versus 2D-Laparoscopic Gastrectomy at 5 Years After Surgery. eFig. 5. Kaplan–Meier Curves Comparing Overall Survival (A) and Disease-free Survival (B) Between the 3D Group and 2D Group in patients with total gastrectomy. eFig. 6. Kaplan–Meier Curves Comparing Overall Survival (A) and Disease-free Survival (B) Between the 3D Group and 2D Group in patients with distal gastrectomy. eFig. 7. Kaplan–Meier Curves Comparing Overall Survival (A) and Disease-free Survival (B) Between the 3D Group and 2D Group in patients of pI staging. eFig. 8. Kaplan–Meier Curves Comparing Overall Survival (A) and Disease-free Survival (B) Between the 3D Group and 2D Group in patients of pII staging. eFig. 9. Kaplan–Meier Curves Comparing Overall Survival (A) and Disease-free Survival (B) Between the 3D Group and 2D Group in patients of pIII staging. eFig. 10. Kaplan–Meier Curves Comparing Overall Survival (A) and Disease-free Survival (B) Between the 3D Group and 2D Group in patients of BMI ≤ 25. eFig. 11. Kaplan–Meier Curves Comparing Overall Survival (A) and Disease-free Survival (B) Between the 3D Group and 2D Group in patients of BMI > 25. (DOCX 1271 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhong, Q., Chen, JY., Shang-Guan, ZX. et al. Long-term oncological outcomes of 3D versus 2D laparoscopic gastrectomy for gastric cancer: a randomized clinical trial. Gastric Cancer 27, 598–610 (2024). https://doi.org/10.1007/s10120-024-01470-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-024-01470-0