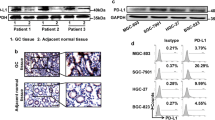
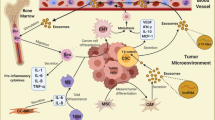
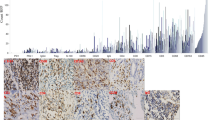
Abstract
Background
Anti-PD-1 immunotherapy has emerged as an important therapeutic modality in advanced gastric cancer (GC). However, drug resistance frequently develops, limiting its effectiveness.
Methods
The role of gastric cancer mesenchymal stem cells (GCMSCs) in anti-PD-1 resistance was evaluated in vivo in NPGCD34+ or NCGPBMC xenograft mouse model. In addition, we investigated CD8+T cell infiltration and effector function by spectral cytometry and IHC. The effects of GCMSCs conditional medium (GCMSC-CM) on GC cell lines were characterized at the level of the proteome, secretome using western blot, and ELISA assays.
Results
We reported that GCMSCs mediated tolerance mechanisms contribute to tumor immunotherapy tolerance. GCMSC-CM attenuated the antitumor activity of PD-1 antibody and inhibited immune response in humanized mouse model. In GC cells under serum deprivation and hypoxia, GCMSC-CM promoted GC cells proliferation via upregulating PD-L1 expression. Mechanistically, GCMSC-derived IL-8 and AKT-mediated phosphorylation facilitated HK2 nuclear localization. Phosphorylated-HK2 promoted PD-L1 transcription by binding to HIF-1α. What is more, GCMSC-CM also induced lactate overproduction in GC cells in vitro and xenograft tumors in vivo, leading to impaired function of CD8+ T cells. Furthermore, CXCR1/2 receptor depletion, CXCR2 receptor antagonist AZD5069 and IL-8 neutralizing antibody application also significantly reversed GCMSCs mediated immunosuppression, restoring the antitumor capacity of PD-1 antibody.
Conclusions
Our findings reveal that blocking GCMSCs-derived IL-8/CXCR2 pathway decreasing PD-L1 expression and lactate production, improving antitumor efficacy of anti-PD-1 immunotherapy, may be of value for the treatment of advanced gastric carcinoma.
Graphical abstract








Similar content being viewed by others
Data availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request, e-mail: zhuwei@ujs.edu.cn.
Abbreviations
- TME:
-
Tumor microenvironment
- PD-1:
-
Programmed death-1
- PD-L1:
-
Programmed death ligand-1
- HK2:
-
Hexokinase II
- PBMCs:
-
Peripheral blood mononuclear cells
- MSCs:
-
Mesenchymal stem cells
- GCMSCs:
-
Gastric cancer mesenchymal stem cells
- BMMSCs:
-
Bone marrow mesenchymal stem cells
- HIF-1α:
-
Hypoxia-inducible factor-1α
- CXCR1:
-
C-X-C motif chemokine receptor 1
- CXCR2:
-
C-X-C motif chemokine receptor 2
- IL-8:
-
Interleukin-8
- IL-1:
-
Interleukin-1
- IFN-γ:
-
Interferon γ
References
Janjigian YY, Bendell J, Calvo E, Kim JW, Ascierto PA, Sharma P, et al. CheckMate-032 study: efficacy and safety of nivolumab and nivolumab plus ipilimumab in patients with metastatic esophagogastric cancer. J Clin Oncol. 2018;36(28):2836–44. https://doi.org/10.1200/JCO.2017.76.6212.
Shitara K, Ozguroglu M, Bang YJ, Di Bartolomeo M, Mandala M, Ryu MH, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392(10142):123–33. https://doi.org/10.1016/S0140-6736(18)31257-1.
Nanda R, Chow LQ, Dees EC, Berger R, Gupta S, Geva R, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J Clin Oncol. 2016;34(21):2460–7. https://doi.org/10.1200/JCO.2015.64.8931.
Wieser V, Adolph TE, Enrich B, Kuliopulos A, Kaser A, Tilg H, et al. Reversal of murine alcoholic steatohepatitis by pepducin-based functional blockade of interleukin-8 receptors. Gut. 2017;66(5):930–8. https://doi.org/10.1136/gutjnl-2015-310344.
Eruslanov EB, Bhojnagarwala PS, Quatromoni JG, Stephen TL, Ranganathan A, Deshpande C, et al. Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J Clin Investig. 2014;124(12):5466–80. https://doi.org/10.1172/JCI77053.
Liu Y, Zhang Y, Wang S, Dong QZ, Shen Z, Wang W, et al. Prospero-related homeobox 1 drives angiogenesis of hepatocellular carcinoma through selectively activating interleukin-8 expression. Hepatology. 2017;66(6):1894–909. https://doi.org/10.1002/hep.29337.
Shang A, Gu C, Zhou C, Yang Y, Chen C, Zeng B, et al. Exosomal KRAS mutation promotes the formation of tumor-associated neutrophil extracellular traps and causes deterioration of colorectal cancer by inducing IL-8 expression. Cell Commun Signal. 2020;18(1):52. https://doi.org/10.1186/s12964-020-0517-1.
Siu MKY, Jiang YX, Wang JJ, Leung THY, Ngu SF, Cheung ANY, et al. PDK1 promotes ovarian cancer metastasis by modulating tumor-mesothelial adhesion, invasion, and angiogenesis via alpha5beta1 integrin and JNK/IL-8 signaling. Oncogenesis. 2020;9(2):24. https://doi.org/10.1038/s41389-020-0209-0.
Schalper KA, Carleton M, Zhou M, Chen T, Feng Y, Huang SP, et al. Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nat Med. 2020;26(5):688–92. https://doi.org/10.1038/s41591-020-0856-x.
Yuen KC, Liu LF, Gupta V, Madireddi S, Keerthivasan S, Li C, et al. High systemic and tumor-associated IL-8 correlates with reduced clinical benefit of PD-L1 blockade. Nat Med. 2020;26(5):693–8. https://doi.org/10.1038/s41591-020-0860-1.
Sun L, Wang Q, Chen B, Zhao Y, Shen B, Wang H, et al. Gastric cancer mesenchymal stem cells derived IL-8 induces PD-L1 expression in gastric cancer cells via STAT3/mTOR-c-Myc signal axis. Cell Death Dis. 2018;9(9):928. https://doi.org/10.1038/s41419-018-0988-9.
Wang Q, Huang C, Ding Y, Wen S, Wang X, Guo S, et al. Inhibition of CCCTC Binding Factor-Programmed Cell Death Ligand 1 Axis Suppresses Emergence of Chemoresistance Induced by Gastric Cancer-Derived Mesenchymal Stem Cells. Front Immunol. 2022;13:884373. https://doi.org/10.3389/fimmu.2022.884373.
Donnelly JM, Engevik AC, Engevik M, Schumacher MA, Xiao C, Yang L, et al. Gastritis promotes an activated bone marrow-derived mesenchymal stem cell with a phenotype reminiscent of a cancer-promoting cell. Dig Dis Sci. 2014;59(3):569–82. https://doi.org/10.1007/s10620-013-2927-z.
Sun L, Wang Q, Chen B, Zhao Y, Shen B, Wang X, et al. Human gastric cancer mesenchymal stem cell-derived IL15 contributes to tumor cell epithelial-mesenchymal transition via upregulation tregs ratio and PD-1 expression in CD4(+)T cell. Stem Cells Dev. 2018;27(17):1203–14. https://doi.org/10.1089/scd.2018.0043.
Chen B, Cai T, Huang C, Zang X, Sun L, Guo S, et al. G6PD-NF-kappaB-HGF signal in gastric cancer-associated mesenchymal stem cells promotes the proliferation and metastasis of gastric cancer cells by upregulating the expression of HK2. Front Oncol. 2021;11:648706. https://doi.org/10.3389/fonc.2021.648706.
Shitara K, Ajani JA, Moehler M, Garrido M, Gallardo C, Shen L, et al. Nivolumab plus chemotherapy or ipilimumab in gastro-oesophageal cancer. Nature. 2022;603(7903):942–8. https://doi.org/10.1038/s41586-022-04508-4.
Gordan JD, Thompson CB, Simon MC. HIF and c-Myc: sibling rivals for control of cancer cell metabolism and proliferation. Cancer Cell. 2007;12(2):108–13. https://doi.org/10.1016/j.ccr.2007.07.006.
Wang ZH, Peng WB, Zhang P, Yang XP, Zhou Q. Lactate in the tumour microenvironment: From immune modulation to therapy. EBioMedicine. 2021;73:103627. https://doi.org/10.1016/j.ebiom.2021.103627.
Thomas GE, Egan G, Garcia-Prat L, Botham A, Voisin V, Patel PS, et al. The metabolic enzyme hexokinase 2 localizes to the nucleus in AML and normal haematopoietic stem and progenitor cells to maintain stemness. Nat Cell Biol. 2022;24(6):872–84. https://doi.org/10.1038/s41556-022-00925-9.
Palsson-McDermott EM, Curtis AM, Goel G, Lauterbach MA, Sheedy FJ, Gleeson LE, et al. Pyruvate kinase M2 regulates Hif-1alpha activity and IL-1beta induction and is a critical determinant of the warburg effect in LPS-activated macrophages. Cell Metab. 2015;21(1):65–80. https://doi.org/10.1016/j.cmet.2014.12.005.
Ippolito L, Morandi A, Giannoni E, Chiarugi P. Lactate: a metabolic driver in the tumour landscape. Trends Biochem Sci. 2019;44(2):153–66. https://doi.org/10.1016/j.tibs.2018.10.011.
Watson MJ, Vignali PDA, Mullett SJ, Overacre-Delgoffe AE, Peralta RM, Grebinoski S, et al. Metabolic support of tumour-infiltrating regulatory T cells by lactic acid. Nature. 2021;591(7851):645–51. https://doi.org/10.1038/s41586-020-03045-2.
Xin Q, Wang H, Li Q, Liu S, Qu K, Liu C, et al. Lactylation: a passing fad or the future of posttranslational modification. Inflammation. 2022;45(4):1419–29. https://doi.org/10.1007/s10753-022-01637-w.
Liu H, Zhao Q, Tan L, Wu X, Huang R, Zuo Y, et al. Neutralizing IL-8 potentiates immune checkpoint blockade efficacy for glioma. Cancer Cell. 2023;41(4):693-710 e8. https://doi.org/10.1016/j.ccell.2023.03.004.
Wang M, Chen B, Sun XX, Zhao XD, Zhao YY, Sun L, et al. Gastric cancer tissue-derived mesenchymal stem cells impact peripheral blood mononuclear cells via disruption of Treg/Th17 balance to promote gastric cancer progression. Exp Cell Res. 2017;361(1):19–29. https://doi.org/10.1016/j.yexcr.2017.09.036.
Wang M, Zhao X, Qiu R, Gong Z, Huang F, Yu W, et al. Lymph node metastasis-derived gastric cancer cells educate bone marrow-derived mesenchymal stem cells via YAP signaling activation by exosomal Wnt5a. Oncogene. 2021;40(12):2296–308. https://doi.org/10.1038/s41388-021-01722-8.
Gao QZ, Cui LJ, Huang C, Zhou CL, Chen B, Wang QQ, et al. Gastric cancer-derived exosomes induce PD-L1 expression on human bone marrow mesenchymal stem cells through the AKT-c-Myc signal axis. All Life. 2022;15(1):442–51. https://doi.org/10.1080/26895293.2022.2058098.
Sun L, Huang C, Zhu M, Guo S, Gao Q, Wang Q, et al. Gastric cancer mesenchymal stem cells regulate PD-L1–CTCF enhancing cancer stem cell-like properties and tumorigenesis. Theranostics. 2020;10(26):11950–62. https://doi.org/10.7150/thno.49717.
Zhai J, Shen J, Xie G, Wu J, He M, Gao L, et al. Cancer-associated fibroblasts-derived IL-8 mediates resistance to cisplatin in human gastric cancer. Cancer Lett. 2019;454:37–43. https://doi.org/10.1016/j.canlet.2019.04.002.
Wu TD, Madireddi S, de Almeida PE, Banchereau R, Chen YJ, Chitre AS, et al. Peripheral T cell expansion predicts tumour infiltration and clinical response. Nature. 2020;579(7798):274–8. https://doi.org/10.1038/s41586-020-2056-8.
Lee YS, Choi I, Ning Y, Kim NY, Khatchadourian V, Yang D, et al. Interleukin-8 and its receptor CXCR2 in the tumour microenvironment promote colon cancer growth, progression and metastasis. Br J Cancer. 2012;106(11):1833–41. https://doi.org/10.1038/bjc.2012.177.
Jeffery CJ. Moonlighting proteins. Trends Biochem Sci. 1999;24(1):8–11. https://doi.org/10.1016/s0968-0004(98)01335-8.
Wang Z, Li M, Jiang H, Luo S, Shao F, Xia Y, et al. Fructose-1,6-bisphosphatase 1 functions as a protein phosphatase to dephosphorylate histone H3 and suppresses PPARalpha-regulated gene transcription and tumour growth. Nat Cell Biol. 2022;24(11):1655–65. https://doi.org/10.1038/s41556-022-01009-4.
Yang W, Xia Y, Hawke D, Li X, Liang J, Xing D, et al. PKM2 phosphorylates histone H3 and promotes gene transcription and tumorigenesis. Cell. 2012;150(4):685–96. https://doi.org/10.1016/j.cell.2012.07.018.
Roberts DJ, Tan-Sah VP, Smith JM, Miyamoto S. Akt phosphorylates HK-II at Thr-473 and increases mitochondrial HK-II association to protect cardiomyocytes. J Biol Chem. 2013;288(33):23798–806. https://doi.org/10.1074/jbc.M113.482026.
Li H, Lu S, Chen Y, Zheng L, Chen L, Ding H, et al. AKT2 phosphorylation of hexokinase 2 at T473 promotes tumorigenesis and metastasis in colon cancer cells via NF-kappaB, HIF1alpha, MMP2, and MMP9 upregulation. Cell Signal. 2019;58:99–110. https://doi.org/10.1016/j.cellsig.2019.03.011.
Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560(7718):382–6. https://doi.org/10.1038/s41586-018-0392-8.
Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–13. https://doi.org/10.1126/science.aan6733.
Acknowledgements
The authors thank AstraZeneca (China) Co. Ltd., for providing AZD5069 drug during the research.
Funding
This work was supported by the National Natural Science Foundation of China No. 81972313 (WZ), No. 81972822 (BS), No. 82203547 (LS), the Bethune Charitable Foundation No. G-X-2019-0101-12 (BS).
Author information
Authors and Affiliations
Contributions
CH and BC: conceptualization, methodology, validation, data curation, formal analysis, writing—original draft preparation. YYZ, QQW, LS, and QZG: validation, formal analysis. ZHC, CLZ, DQW, JX, XW: resources. MW, XZ and WRX: conceptualization. WZ and BS: writing—review and editing, supervision, funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest.
Ethical approval
This study involves human subjects, and all samples were obtained following individual informed consent and ethical approval by the Ethics Committee of the Affiliated Cancer Hospital of Nanjing Medical University ID#: 2021ke-010. The subjects gave informed consent to participate in the study before taking part. The animal study was approved by the Ethics Committee of Jiangsu University. All procedures were conducted following the guidelines of the National Institute of Health regarding the care and use of laboratory animals (NIH Publication No. 8023, revised 1978) ID#: UJS-IACUC-AP-2022022812.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, C., Chen, B., Wang, X. et al. Gastric cancer mesenchymal stem cells via the CXCR2/HK2/PD-L1 pathway mediate immunosuppression. Gastric Cancer 26, 691–707 (2023). https://doi.org/10.1007/s10120-023-01405-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-023-01405-1