Abstract
Background
Splenectomy for dissecting splenic hilar lymph nodes (#10) should be avoided for most gastric cancer, considering the high morbidity and lack of any survival benefit, but it is often selected for scirrhous gastric cancer because this type frequently invades the whole stomach and lymph nodes. Splenectomy is necessary for dissecting #10; however, the survival benefit of dissecting #10 is unclear.
Methods
Patients who had scirrhous gastric cancer and underwent D2 total gastrectomy with splenectomy at National Cancer Center Hospital, Japan, between 2000 and 2011 were retrospectively analyzed. The therapeutic value index was calculated by multiplying the metastatic rate of each nodal station and the 5-year survival of patients who had metastasis to each node.
Results
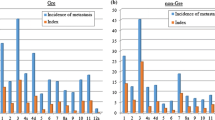
In total, 137 patients were eligible for the present study. The most frequent metastatic node was #3(58%), followed by #4d(46%), #1(35%), #4sb(23%), #6(22%), #7(21%), #4sa(18%), #10(15%), #2(14%), #11p(14%), #11d(13%), #9(13%), and #8a(11%). These lymph nodes had a metastatic rate of more than 10%. The node station with the highest index was #3(18.9), followed by #4d(14.1), #1(10.8), #4sa(6.11), #4sb(6.06), #10(5.09), #7(4.39), #11d(4.36), #11p(4.06), #2(2.93), #8a(2.18), and #9(1.45). The index of #10 exceeded that of #2, #7, #8a, and #9, which are the key nodes dissected in D2.
Conclusion
The metastatic rate of the splenic hilar lymph nodes was relatively high, and the therapeutic index was the sixth highest among the 15 regional lymph nodes included in D2 dissection. Splenectomy for dissecting splenic hilar lymph nodes would be justified for scirrhous gastric cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Scirrhous gastric cancer, also recognized as Borrmann type 4 or linitis plastica, is an uncommon type of gastric cancer that is diffusely infiltrative toward the entire stomach involving the gastric serosa and lymph nodes [1, 2]. Accordingly, patients with scirrhous gastric cancer are frequently accompanied by peritoneal seeding and gross lymph node metastasis [1, 3] and have a poor prognosis despite advances in surgery and chemotherapy [3,4,5,6].
Radical gastrectomy with D2 lymph node resection has been accepted as a standard surgery for locally advanced gastric cancer, regardless of the type [7]. Classical D2 total gastrectomy includes splenectomy for complete dissection of splenic hilar lymph nodes; however, splenectomy is reportedly a risk factor for mortality in Western countries [8] and for morbidity in Japan as well [9]. Spleen-preserving D2 without dissecting the splenic hilar lymph nodes was thus developed, and its non-inferiority was confirmed in a Japanese phase III trial (JCOG0110) [9]. However, that trial excluded tumors invading the greater curvature and scirrhous gastric cancer, as these tumors were believed to have highly involved the splenic hilar lymph nodes. It therefore has been unclear whether or not splenic hilar lymph nodes should be dissected for these tumors.
The efficacy of dissection was able to be evaluated based on the proportion of patients who had nodal metastasis and survived for more than 5 years thanks to nodal dissection. This theory was proposed by Sasako [10] and was named the therapeutic index. Recently, we revealed that non-scirrhous gastric cancer invading the greater curvature had a relatively high therapeutic index at the splenic hilar nodes [11], suggesting that the splenic hilar lymph nodes should be dissected for these tumors. However, whether or not this holds true for scirrhous gastric cancer has been unclear. In the scirrhous gastric cancer, frequent nodal metastasis may work to increase the therapeutic index, but frequent peritoneal recurrence may offset the efficacy of nodal dissection.
Given the above, the present study aimed to evaluate the therapeutic index of splenic hilar lymph node in scirrhous gastric cancer to investigate whether or not dissecting splenic hilar lymph nodes is justified.
Patients and methods
Patients
The patients were selected from the clinical database of consecutive patients who underwent gastrectomy at the National Cancer Center Hospital from January 2000 to December 2011, according to the following criteria: (1) preoperative diagnosis of scirrhous gastric cancer was defined as type 4 gastric cancer which was basically determined following the latest Japanese Classification of Gastric Carcinoma available for each patient after discussion of surgeon and endoscopist by reviewing the endoscopy and computed tomography, (2) underwent D2 total gastrectomy with splenectomy, and (3) achieved R0 resection or R1 only based on positive peritoneal cytology (CY1).
Surgical procedure and follow-up
In our hospital, splenectomy to dissect the splenic hilar nodes was a standard procedure and was routinely selected for scirrhous gastric cancer. D2 total gastrectomy with splenectomy was performed as in JCOG0110 [9]; the body and tail of the pancreas were fully mobilized from the retroperitoneum, and the spleen was removed en bloc with the hilar nodes following dissection of proximal splenic artery lymph nodes (#11p) and distal splenic artery lymph nodes (#11d). Postoperative chemotherapy was administered according to the physician’s decision, considering the final tumor stage and general condition of the patient. Since 2007, patients diagnosed with stage II/III (except for T1N2-3/T3N0) have received S-1 adjuvant chemotherapy for 1 year after surgery as long as feasible, and patients diagnosed with stage IV have received chemotherapy, but the duration was determined by physician’s choice for as long as feasible. Outpatient follow-up was continued for at least 5 years.
Therapeutic value index
The regional lymph nodes were categorized into the station according to the JGCA classification [12]. The incidence of lymph node metastasis was determined by referencing the pathological reports for each lymph node station. The therapeutic value index was calculated by multiplying the frequency of metastasis to the station by the 5-year survival rate of patients with metastasis to each station, as previously reported [10].
Classifications and statistical analyses
Tumor stages were classified by the 7th edition of the TNM, and the D number was determined by the 4th edition of the Japanese gastric cancer treatment guidelines [7].
All statistical analyses were performed using the SPSS software program, version 24 (Statistical Package for the Social Sciences; SPSS, Chicago, IL, USA). The overall survival (OS) was defined as the period from the date of surgery to the date of death from any cause. The data of the patients who did not experience an event were censored on the date of the final observation. The survival data were obtained from hospital records. Survival curves were constructed by the Kaplan–Meier method.
This study was approved by the Institutional Review Board (IRB) of the National Cancer Center (2017 epidemiologic study-22).
Results
Patient’s demographics
Among the 312 patients who underwent gastrectomy for scirrhous gastric cancer, a total of 137 were included in the present study. Figure 1 shows the consort diagram of the present study. Table 1 summarizes the patient demographics and tumor characteristics. In summary, most tumors had a maximal diameter of more than 10 cm, and the vast majority of patients had serosa invasion (pT3 or pT4) with histologically undifferentiated-type tumors. Similarly, predominant tumor location was mostly in the middle body and upper body of the stomach. Lymph node metastasis was observed in 96 (71%) patients. CY1 was found in 16 patients (11%) and classified as stage IV.
Survival outcomes
The median survival time of all patients was 46.1 months (95% confidence interval [CI]: 38.2–54.0 months). Figure 2 shows the survival curve. The 5-year OS rate was 38% in overall patients and 50.0% in Stage I, 66.2 in Stage II, 30.8 in Stage III, and 9.4% in Stage IV disease.
Lymph node metastasis and the therapeutic value index
Table 2 shows the metastatic rate of each nodal station, the 5-year survival rates in patients who had metastasis to each station, and the therapeutic index of each station. The most frequent metastatic site was the lesser curvature lymph nodes (#3), followed by the right greater curvature lymph nodes (#4d), the right paracardial lymph nodes (#1), the left greater curvature lymph nodes along the left gastroepiploic artery (#4sb), the infrapyloric lymph nodes (#6), the lymph nodes along the trunk of left gastric artery (#7), the left greater curvature lymph nodes along the short gastric artery (#4sa), the left paracardial lymph nodes (#2), the splenic hilar nodes (#10), the distal splenic artery lymph nodes (#11d), the proximal splenic artery lymph nodes (#11p), the celiac artery lymph nodes (#9), and the anterosuperior lymph nodes along the common hepatic artery (#8a). These lymph nodes had a metastatic rate of more than 10%. The node with the highest index was #3, followed by #4d, #1, #4sa, #4sb, #10, #7, #11d, #11p, #2, #8a, and #9. The index of #10 was higher than that of #2, #7, #8a, and #9 which are the key nodes dissected in D2.
Discussion
Scirrhous gastric cancer is characterized by diffuse infiltration, frequent peritoneal metastasis, and a poor prognosis, differing completely from the non-scirrhous gastric cancer. In the present study, we examined the survival impact of dissection of splenic hilar lymph nodes (#10) by calculating the therapeutic index in scirrhous gastric cancer and found that the metastatic rate and therapeutic index of #10 were the eighth and sixth highest, respectively, among the 15 regional lymph nodes dissected by D2 gastrectomy. Furthermore, the index was higher than any other second-tier nodal station. These results suggest that dissection of the splenic hilar lymph nodes is justified for scirrhous gastric cancer.
In the present study, the metastatic rate to #10 was 15.3%. No previous report has examined the incidence of nodal metastasis by focusing on the scirrhous gastric cancer. Regarding tumors invading the greater curvature, previous studies have reported metastatic rates to #10 of 13.4% [13] and 15.9% [14] including scirrhous and non-scirrhous gastric cancer and 15.9% when limited to the non-scirrhous gastric cancer [11]. Thus, the metastatic rate to #10 is similar between the scirrhous and the non-scirrhous gastric cancer invading the greater curvature. However, the metastatic rate to #10 was only 2.4% for tumors not invading the greater curvature [9]. Metastasis to #10 therefore appears to be determined based solely on whether or not the tumor invades the greater curvature.
Whether or not a nodal station should be dissected is determined not only by the metastatic rate, but also by the survival benefit. Therefore, we calculated the therapeutic index, which reflects the proportion of patients who had nodal metastasis but survived for more than 5 years after its nodal dissection. In the present study, the therapeutic index of #10 was sixth highest among the 15 nodes dissected. No previous report had clarified the index in scirrhous gastric cancer. Previous reports examining the tumors invading the greater curvature found that the therapeutic index of #10 was ninth [13] and fourth highest [14] in both of scirrhous and non-scirrhous gastric cancer and eighth highest when limited to non-scirrhous gastric cancer [11]. In all reports, including the present study, the index was higher than in the nodes along the common hepatic artery and splenic artery. Therefore, the priority of splenic hilar nodal dissection would be similar, regardless of scirrhous or non-scirrhous gastric cancer.
The prognosis of scirrhous gastric cancer has historically been believed to be extremely poor. Schauer et al. [5] reported that scirrhous gastric cancer was not a disease that could be managed surgically. In the 1990s, the 5-year OS was reported to be 8–13% [3] with surgery alone. In the present study, however, the 3-year OS was around 60%, and the 5-year OS exceeded 40%, which was higher than expected. This marked discrepancy in the survival can be explained by several reasons, such as the exclusion of high-risk patients by peritoneal cytology, the low rate of complications due to sophisticated D2 surgery and perioperative care, and effective adjuvant chemotherapy. Although the absolute index of #10 was slightly lower in the scirrhous gastric cancer than in the non-scirrhous gastric cancer, the survival benefit dissecting splenic hilar lymph nodes would be justified, considering the relatively high 3- and 5-year OS.
Several points which should be considered when interpreting the results of the present study. First, this study was limited to patients who had undergone total gastrectomy with splenectomy. As the patients who were less tolerable of this surgery were necessarily excluded, the index may have been overestimated. Therefore, the applicability to dissect splenic hilar lymph nodes must be carefully determined in each patient. Second, postoperative adjuvant chemotherapy was administered to only 60 (42%) patients. However, the index would be underestimated if adjuvant chemotherapy were effective, so the conclusion of the present study is not affected by this point. Similarly, nearly one-third of patients received neoadjuvant chemotherapy, which could have effect on the incidence of lymph node metastasis and the therapeutic index. However, a pathological complete response of the lymph nodes was not induced by neoadjuvant chemotherapy selectively at the locations of lymph nodes. Thus, the order of therapeutic index might not be changed by the impact of neoadjuvant chemotherapy. Third, the therapeutic index does not consider factors that might influence the overall survival. The patient characteristics, tumor factors, and adjuvant treatment completion all differed among patients. A randomized controlled trial comparing splenectomy and non-splenectomy will be necessary to confirm the survival benefit dissecting splenic hilar lymph nodes for patients with scirrhous gastric cancer. Fourth, splenectomy for dissecting splenic hilar nodes easily causes morbidity, which would worsen the prognosis and decrease the index of splenic hilar lymph nodes. In this study, morbidity (> grade II) was observed in 20 patients (15%). In these patients, #10 nodal metastasis was found in two patients (10%), but both the patients could not survive for more than 5 years. Thus, the therapeutic index of #10 was 0 when morbidity occurred. Therefore, splenectomy for dissecting splenic hilar lymph nodes must be avoided for the patients who have high risk of morbidity.
In conclusion, scirrhous gastric cancer had relatively high rates of metastasis to the splenic hilar lymph nodes and a high therapeutic index. The splenic hilar lymph nodes have priority among the regional lymph nodes included in D2 lymphadenectomy, and dissection of the splenic hilar lymph nodes for patients with scirrhous gastric cancer is justified.
References
Kitamura K, Beppu R, Anai H, Ikejiri K, Yakabe S, Sugimachi K, et al. Clinicopathologic study of patients with Borrmann type IV gastric carcinoma. J Surg Oncol. 1995;58(2):112–7.
Kodera Y, Ito S, Mochizuki Y, Yamamura Y, Misawa K, Ohashi N, et al. The number of metastatic lymph nodes is a significant risk factor for bone metastasis and poor outcome after surgery for linitis plastica-type gastric carcinoma. World J Surg. 2008;32(9):2015–20.
Otsuji E, Kuriu Y, Okamoto K, Ochiai T, Ichikawa D, Hagiwara A, et al. Outcome of surgical treatment for patients with scirrhous carcinoma of the stomach. Am J Surg. 2004;188(3):327–32.
Suga S, Iwase H, Shimada M, Nishio Y, Ichihara T, Ichihara S, et al. Neoadjuvant chemotherapy in scirrhous cancer of the stomach using uracil and tegafur and cisplatin. Intern Med (Tokyo, Japan). 1996;35(12):930–6.
Schauer M, Peiper M, Theisen J, Knoefel W. Prognostic factors in patients with diffuse type gastric cancer (linitis plastica) after operative treatment. Eur J Med Res. 2011;16(1):29–33.
Blackham AU, Swords DS, Levine EA, Fino NF, Squires MH, Poultsides G, et al. Is linitis plastica a contraindication for surgical resection: a multi-institution study of the US Gastric Cancer Collaborative. Ann Surg Oncol. 2016;23(4):1203–11.
JGCA. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20(1):1–19.
Cuschieri A, Weeden S, Fielding J, Bancewicz J, Craven J, Joypaul V, et al. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer. 1999;79(9–10):1522–30.
Sano T, Sasako M, Mizusawa J, Yamamoto S, Katai H, Yoshikawa T, et al. Randomized controlled trial to evaluate splenectomy in total gastrectomy for proximal gastric carcinoma. Ann Surg. 2017;265(2):277–83.
Sasako M, McCulloch P, Kinoshita T, Maruyama K. New method to evaluate the therapeutic value of lymph node dissection for gastric cancer. Br J Surg. 1995;82(3):346–51.
Yura M, Yoshikawa T, Otsuki S, Yamagata Y, Morita S, Katai H, et al. The therapeutic survival benefit of splenic hilar nodal dissection for advanced proximal gastric cancer invading the greater curvature. Ann Surg Oncol. 2019;26(3):829–35.
JGCA. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14(2):101–12.
Maezawa Y, Aoyama T, Yamada T, Kano K, Hayashi T, Sato T, et al. Priority of lymph node dissection for proximal gastric cancer invading the greater curvature. Gastric Cancer. 2018;21(3):569–72.
Watanabe M, Kinoshita T, Enomoto N, Shibasaki H, Nishida T. Clinical significance of splenic hilar dissection with splenectomy in advanced proximal gastric cancer: an analysis at a single institution in Japan. World J Surg. 2016;40(5):1165–71.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest in association with the present study.
Ethical standards
This study was conducted with the approval of the National Cancer Center Hospital Ethics Committee (No.: 2017-077).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hayashi, T., Yoshikawa, T., Kamiya, A. et al. Is splenectomy for dissecting splenic hilar lymph nodes justified for scirrhous gastric cancer?. Gastric Cancer 23, 922–926 (2020). https://doi.org/10.1007/s10120-020-01063-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-020-01063-7