Abstract
Background
An increase of regulatory T cells, defined as CD25high- and/or FOXP3+-expressing CD4+ T cells, within tumors has been reported in several studies. Tregs promote tumor growth by modulating the antitumor immune response, mainly through inhibition of T-cell-mediated tumor cell killing: this has been suggested to be dependent on IL-10 and/or TGF-β. In stomach cancer, the mechanisms behind the accumulation of Tregs in tumor tissue has not been fully elucidated, and neither has Treg gene expression in situ.
Materials and methods
Stomach tissue from gastric cancer patients undergoing gastric resection was analyzed using flow cytometry and cell sorting, followed by RT-PCR.
Results
We observed that stomach CD4+ FOXP3+ T cells proliferated to a higher degree than CD4+ FOXP3− T cells, which may contribute to Treg accumulation in the mucosa. By analyzing DNA methylation, we demonstrated that both proliferating and nonproliferating FOXP3+ T cells exhibited complete demethylation of the FOXP3 gene, indicating a stable FOXP3 expression in both cell populations. Furthermore, analysis of T-cell populations isolated directly from the tumor and tumor-free mucosa demonstrated that CD4+ CD25high T cells have a higher IL-10/IFN-γ gene expression ratio but express lower levels of TGF-β than CD4+ CD25low/− T cells.
Conclusion
We demonstrate strong proliferation among regulatory CD4+ FOXP3+ CD25high T cells in the gastric cancer mucosa. These local Treg express a suppressive cytokine profile characterized by high IL-10 and low TGF-β and IFN-γ production.
Similar content being viewed by others
Introduction
Gastric adenocarcinoma is the third most common cause of cancer death worldwide, after lung cancer and liver cancer [1]. Although an unhealthy diet and smoking increase risk, the main risk factor for developing gastric cancer is infection with the bacterium Helicobacter pylori [2]. The mechanism by which H. pylori causes cancer is not fully understood, but it is currently believed to be mediated by the active chronic inflammation induced by the bacterium, and the process is dependent on certain combinations of bacterial pathogenicity, host susceptibility, and environmental factors [3]. An important carcinogenic component of H. pylori is the oncoprotein cagA, which is translocated into gastric epithelial cells where it alters host intracellular signaling [4]. Translocated cagA is rapidly degraded in normal gastric epithelial cells, but it may be retained in its active form in gastric stem cells and thereby increase the risk of carcinogenicity [5].
For a tumor to become established, the tumor cells need to find a means to evade the immune system to avoid destruction by cell-mediated immune mechanisms [6]. This immune evasion may occur in different ways, including the production of inhibitory cytokines and metabolites such as interleukin (IL)-10, transforming growth factor (TGF)-β, and prostaglandins by the tumor cells themselves or by macrophages infiltrating the tumor [7]. In recent years, recruitment of regulatory T cells (Tregs) within tumors has been demonstrated to be an immune evasion mechanism [8, 9]. Increased numbers of regulatory T cells have been shown in the tumor mucosa of many cancer types, including ovarian cancer, small cell lung cancer, breast cancer, colorectal cancer, and gastric cancer [10–12], and Tregs have been associated with poor outcomes in gastric cancer [13, 14].
Tregs are generally characterized by expression of CD4, of high levels of CD25 (CD25high), and of the transcription factor FOXP3, which has been linked to suppressive activity in both humans and mice [15, 16]. Initial studies of CD4+ CD25high FOXP3+ Treg reported that the regulatory cells only originate from the thymus and then migrate to the periphery, but more recently it was shown that FOXP3 expression may also be induced in non-Treg (CD4+ CD25−) cells [17–19]. We have previously shown that individuals with gastric cancer or H. pylori infection have higher levels of CD4+CD25high Treg in the mucosa [20, 21] and that the frequency of FOXP3-expressing T cells is increased in the gastric tumor mucosa compared to tumor-free gastric mucosa [22]. The increase in FOXP3-expressing Tregs in tumor mucosa may be caused by increased recruitment of CCR4-expressing Tregs into the tumor [22, 23]. However, in light of the finding that FOXP3 expression may be induced in the periphery, an alternative explanation to the increased numbers of Tregs in tumors could be that CD4+ CD25− non-Treg cells are induced to express FOXP3 in the mucosa. There is also a possibility that the increased numbers of FOXP3-expressing cells are caused by induced proliferation of Tregs within the tumor; in line with this, it was recently shown that hepatic cancer cells cultured together with peripheral blood mononuclear cells (PBMC) promote CD4+ CD25high T-cell proliferation and inhibit CD4+ CD25− T-cell proliferation [24].
In this study, we investigated whether local proliferation of Treg in the tumor mucosa may be a putative explanation for the higher number of Tregs observed. Furthermore, we confirmed the suppressive phenotype of the Treg cells from the gastric mucosa by studying CTLA-4 and CD27 expression in CD4+ FOXP3+ cells as well as by analyzing cytokine gene expression of sorted CD4+ CD25high T cells.
Methods
Subjects and sample collection
Thirteen patients with non-cardia gastric adenocarcinoma (median age, 78 years; age range, 63–85 years; 4 females) undergoing total or partial distal gastrectomy at Sahlgrenska University Hospital were included in the study. Patients with gastric cardia adenocarcinoma were excluded because this subtype of gastric cancer is not related to infection with H. pylori [25]. A blood sample as well as resection material of gastric tumor and tumor-free (defined as tissue with normal visual appearance located at least 5 cm from the nearby tumor) mucosa were collected from each patient. Five persons (median age, 68 years; age range, 57–75 years; 3 females) without diagnosed gastric cancer were used as healthy controls; from these controls, only blood samples were collected.
The resection material was used for isolation of lamina propria lymphocytes and for RNA extraction. For each patient, the H. pylori status was evaluated by different tests, including culture on Columbia-Iso agar plates, quantitative real-time polymerase chain reaction (PCR) [26], and serology tests, where H. pylori status was determined by in-house enzyme-linked immunoassay (ELISA) methods [27], as well as with an EIA-G III ELISA kit (Orion Diagnostics, Finland) and summarized in Table 1. There were no differences in any of the subsequent analyses between H.p. + and H.p. −; therefore, all cancer patients were grouped together in the figures. The study was approved by the human research ethics committee at University of Gothenburg, and informed consent was obtained from each volunteer before participation. None of the patients had undergone radiotherapy or chemotherapy before gastrectomy.
Isolation of lymphocytes and flow cytometry analysis
Lamina propria lymphocytes (LPL) were isolated using collagenase/DNAse enzymatic digestion, as previously described [28]. From heparinized venous blood, peripheral blood mononuclear cells (PBMC) were isolated by density-gradient centrifugation on Ficoll-Pague (GE Healthcare Bio-Sciences, Sweden). The cells were stained for cell-surface expression using optimal concentrations of anti-CD4-FITC, anti-CD8-PerCP, anti-CD25-PE, or anti-CD27-PE antibodies followed by intracellular staining of FOXP3-APC (antibody clone PCH101) and/or Ki-67-PE or CTLA-4-PE using permeabilization buffer (eBioscience, San Diego, CA, USA) according to the protocol of the manufacturer. After staining, the cell were fixed in formaldehyde and analyzed by flow cytometry using a FACSCalibur equipped with a blue and a red laser (BD, San Jose, CA, USA). Because of criticism of the anti-FOXP3 antibody clone PCH101 [29], we first confirmed that PCH101 yielded identical staining as the clone 259D in our ex vivo samples [30].
RNA isolation
The mucosal tissue material collected from gastric cancer patients was immediately placed in RNAlater (Ambion) and stored at −70 °C until extraction of RNA. Isolated LPL and PBMC were sorted into CD4+ CD25high and CD4+ CD25low/− T cells using a FACSVantage SE (BD Bioscience) as previously described [20]. The cells were placed in lysis buffer with 1 % β-mercaptoethanol and stored at −70 °C until extraction of RNA. Total RNA from equal numbers of sorted CD4+ CD25high and CD4+ CD25low/− T cells were extracted from each patient (10,000–50,000 cells per patient), using the RNeasy Micro Kit (Qiagen, Hilden, Germany) according to the description of the manufacturers, and kept at −70 °C until further use.
Quantitative real-time RT-PCR
Preparation of cDNA from RNA of sorted cells was performed by using the Sensiscript Kit (Qiagen) and random hexamers. Taqman gene expression assays for IL-10, TGF-β, interferon (IFN)-γ, and HPRT1 were purchased from Applied Biosystems. The real-time PCR reactions were run in 96-well plates using the standard amplification conditions described for the 7500 Real Time PCR System (Applied Biosystems); each reaction contained 2 μl cDNA, 1× Universal PCR Master Mix, 1 μl primer mix, and ribonuclease-free water in a total volume of 20 μl. All reactions were run in duplicate and the average Ct value for each sample was calculated. Relative quantification was done using HPRT as the housekeeping gene [31]. A calibrator sample was included and run on each individual plate to allow comparisons between results obtained from different runs.
DNA isolation and methylation analysis
Isolated PBMC were stained for flow cytometry analysis and sorted using a FACSAria cell sorter into CD4+ FOXP3+ Ki67+, CD4+ FOXP3+ Ki67−, and CD4+ FOXP3− populations. Genomic DNA from these cell populations was isolated and bisulfite converted using an EZ DNA methylation kit (ZYMO Research, Orange, CA, USA) according to the instructions of the manufacturer. The FOXP3 promoter region was amplified by PCR using forward primer 5′-TGGTGAAGTGGATTGATAGAAAAGG-3′ and reverse primer 5′-TATAAAAACCCCCCCCCACC-3′ at an annealing temperature of 58 °C. The PCR products were then subjected to methylation-sensitive single-NUcleotide primer-extension analysis (Ms-SNUPE) by using an ABI prism SNaP shotTM multiplex kit (Applied Biosystems) according to the manufacturer instructions. The primer used for Ms-SNUPE analysis was 5′-AAAAAAATTTAAATTATTAAAAAAAAAAAATCTAC-3′. Finally, the samples were subjected to capillary electrophoresis on an ABI prism 3730 genetic analyzer, and the corresponding peaks were analyzed by using the Gene mapper V 3.7 software.
Statistical analysis
Differences between the groups were assessed using the Mann–Whitney or Wilcoxon tests, as described in the figure legends.
Results
CD25high FOXP3+ CD4+ T cells increase in the gastric tumor mucosa
First, using flow cytometry we confirmed our previous findings that the frequency of FOXP3+ cells increases in gastric tumor mucosa. Thus, tumor cells showed a twofold increase in CD4+ FOXP3+ T cells compared to the adjacent tumor-free mucosa (p = 0.002) (Fig. 1a). The location of the tumor (antrum/corpus) did not influence the level of FOXP3+ cells (data not shown). Human CD4+ can be divided into three different subpopulations with respect to intensity of CD25 expression: CD25high, CD25low, and CD25neg; this was also true for mucosal FOXP3-expressing CD4+ T cells (Fig. 1b). In tumor and tumor-free mucosa, 50–60 % of FOXP3+ cells were CD25low and 20–25 % were CD25neg (not shown). The CD25high subpopulation, which was 15 % of FOXP3+ cells in tumor-free mucosa, increased twofold in tumor tissue (p = 0.001) (Fig. 1c). Thus, in tumors the total number of FOXP3+ cells increased, as well as the relative frequency of CD25high cells within the FOXP3 population, demonstrating an increased number of Tregs in gastric tumor tissue.
Analysis of FOXP3, CD25, and CD8 in gastric and peripheral blood T cells of gastric cancer patients. Isolated lamina propria lymphocytes from tumor and tumor-free gastric mucosa, and peripheral blood mononuclear cells (PBMC), were stained with anti-CD4-FITC, anti-CD25-PE, anti-CD8-PerCP, and anti-FOXP3-APC, and analyzed by flow cytometry. a Frequencies of FOXP3+ cells among CD4+ T cells. b Contour plots show CD25 and FOXP3 expression among CD4 T cells in one representative subject of nine. The three central rectangles show CD25−, CD25low, and CD25high FOXP3+ cells, respectively. c Frequencies of CD25high FOXP3+ T cells. d Fractions of the FOXP3+ T-cell frequencies divided by the CD8 T-cell frequencies. In the box plots, the horizontal line represents the median, the box edges show the interquartile range (first and third quartile), and the whiskers represent maximum and minimum values, with the exception of outliers. If present, outliers (>1.5 × interquartile range) are shown as dots. In all cases, n = 9 patients were tested; p values refer to Mann–Whitney tests
Gastric tumor mucosa has an increased Treg to CD8 ratio
The presence of cytotoxic CD8 T cells is associated with increased survival in colon cancer [32], and strong antitumor CD8 T-cell response would be expected to be beneficial for survival in gastric cancer. We therefore determined the relationship between CD8 T cells and CD4+ FOXP3+ T cells in the mucosa by flow cytometry. We found that there was a threefold increase of CD4+ FOXP3+ T cells relative to CD8+ T cells in the tumor mucosa compared to tumor-free mucosa, in which there was a similar ratio as in the peripheral blood (p = 0.008) (Fig. 1d). This finding indicates that there is a high degree of immunosuppressive activity relative to the antitumor defense capacity in tumor tissue.
Increased proliferation in the gastric tumor mucosa of CD4+ FOXP3+ T cells
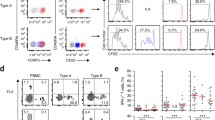
The mechanisms of increased Treg numbers in tumor tissue are still incompletely understood. Flow cytometry analysis of proliferation by Ki-67 staining revealed that there was a high proportion of proliferating cells among CD4+ FOXP3+ T cells. In the tumor mucosa, the proliferation levels were twofold higher among both CD4+ FOXP3+ and CD4+ FOXP3− cells compared to tumor-free mucosa (p = 0.0004) (Fig. 2a, b). FOXP3+ cells exhibited the highest proliferation levels of the two T-cell populations: there was a twofold higher proliferation among FOXP3+ T cells compared to FOXP3− in both tumor-free and tumor mucosa (p = 0.0004) (Fig. 2b). Furthermore, in peripheral blood both gastric cancer patients and healthy controls showed increased proliferation among CD4+ FOXP3+ cells (Fig. 2c).
Analysis of proliferation among gastric and peripheral blood T-cell populations. Isolated lamina propria lymphocytes from tumor and tumor-free gastric mucosa, and PBMCs, were stained with anti-CD4-FITC, anti-Ki67-PE/isotype-ctrl-PE, and anti-FOXP3-APC, and analyzed by flow cytometry. a Contour plots show anti-Ki67-PE (left) or isotype-ctrl-PE (right) staining of CD4+ gastric tumor T cells of one representative individual of eight. b Frequencies of Ki67 expression among FOXP3+ and FOXP3− CD4 T cells from tumor and tumor-free (T-free) mucosa of gastric cancer patients. c Frequencies of Ki67 expression among FOXP3+ and FOXP3− CD4 T cells from peripheral blood of gastric cancer patients (GC) or healthy controls. In the box plots, the horizontal line represents median, the box edges show the interquartile range (first and third quartile), the whiskers depict maximum and minimum values, with the exception of outliers. Existing outliers (>1.5 × interquartile range) are shown as dots. For gastric cancer patients, n = 8; for healthy controls, n = 3. p values refer to Mann–Whitney tests for comparisons between tumor and tumor-free mucosa. For comparisons of FOXP3+ and FOXP3− populations, p values refer to Wilcoxon signed-rank tests
The DNA CpG methylation of the FOXP3 gene of CD4+ FOXP3+ is similar regardless of proliferation status
FOXP3 expression as a marker for a regulatory T-cell phenotype has been subject to criticism because conventional CD4+ effector T cells transiently express FOXP3 upon TCR stimulation [19, 33, 34]. Several publications have shown that CpG demethylation of regulatory elements in the FOXP3 locus support stable expression of FOXP3 and a suppressive phenotype [33, 34]. To address whether proliferating CD4+ FOXP3+ T cells were in fact stably expressing FOXP3 or were activated T cells that transiently expressed FOXP3, we analyzed the CpG methylation status of the promoter region of the FOXP3 gene in both proliferating and nonproliferating T-cell populations. Because of the lack of sufficient T-cell numbers, this analysis was performed on peripheral blood T-cell populations.
The results showed that although there was about 35 % demethylation of the FOXP3 promoter in FOXP3− T cells, both Ki-67+ and Ki-67− CD4+ FOXP3+ T cells exhibited an almost complete demethylation (>95 % demethylation) (Fig. 3). Therefore, there was no difference in the methylation pattern between proliferating and nonproliferating FOXP3+ T cells, which indicates that even proliferating FOXP3+ T cells are Tregs that are expressing FOXP3 in a stable fashion.
Analysis of demethylation of the FOXP3 gene. Isolated peripheral blood CD4 T cells were sorted into FOXP3+ Ki67+, FOXP3+ Ki67−, and FOXP3− Ki67− populations by flow cytometry. Genomic DNA was isolated, and CpG methylation of the FOXP3 promoter was analyzed by methylation-sensitive single-nucleotide primer extension. Results are expressed as percent demethylation of the −77 position of the FOXP3 gene (relative to the transcription start site). As the spread between data points was very low, each individual (n = 3) is shown as a black dot
CD4+ FOXP3+ T cells in gastric cancer mucosa coexpress CTLA-4 and CD27, indicating a suppressive phenotype
FOXP3 has been linked to a suppressive T-cell phenotype [18], but it is not possible to isolate human CD4+ FOXP3+ T cells by cell sorting and use them directly for suppression assays, because intracellular FOXP3 staining kill the cells in the process. We have previously shown that 90 % of the CD4+ CD25high cells in gastric tumor mucosa express high levels of FOXP3 and that this cell population is suppressive [22]. To further analyze the suppressive phenotype of the gastric FOXP3+ T cells, we assessed their expression of CTLA-4 and CD27, as these markers are linked to suppressive activity [35–37]. We confirmed that 85 % of the analyzed FOXP3-expressing cells in the tumor mucosa coexpressed CD27 and nearly 100 % coexpressed CTLA-4 (Fig. 4a–d). Thus, the majority of CD4+ FOXP3+ T cells in the tumor and tumor-free gastric mucosa have a suppressive phenotype. In addition, about 60 % of FOXP3 cells also expressed CTLA4 in tumor tissue, compared to 15 % in blood (not shown), demonstrating that in tumor tissue even FOXP3− T cells may have a suppressive phenotype.
Analysis of CD27 and CTLA4 expression of FOXP3+ CD4 T cells. Isolated lamina propria lymphocyte from tumor and tumor-free gastric mucosa, and PBMC, were stained with anti-CD4-FITC, anti-CD27-PE, or anti-CTLA4-PE and anti-FOXP3-APC, and analyzed by flow cytometry. a, b Contour plots show anti-FOXP3 and anti-CD27 (a) or anti-CTLA4 (b) staining of CD4+ blood or gastric T cells of one representative individual of five (a) or three (b). c Frequencies of CD27 expression of FOXP3+ CD4 T cells. In the box plot, the horizontal line represents median, the box edges show the interquartile range (first and third quartile), and the whiskers depict maximum and minimum values. d Frequencies of CTLA4 expression of FOXP3+ CD4 T cells. Results from all individuals are shown as dots; medians are indicated by horizontal lines
CD4+ CD25high gastric T cells produce IL-10, but CD25low/− cells have a mixed phenotype producing both suppressive and inflammatory cytokines
Finally, to investigate the cytokine expression of the gastric Treg, we performed RT-PCR analysis of mucosal T cells sorted into CD4+ CD25high and CD4+ CD25low/− populations. To preserve RNA quality when using small amounts of mucosal T cells, we chose to use non-fixed cells. We therefore sorted Treg based on CD25 instead of intracellular FOXP3 expression. We found that CD4+ CD25high T cells of both tumor and tumor-free mucosa expressed high levels of IL-10 (5-fold and 25-fold higher than PBMC CD25high cells, respectively) (Fig. 5a), but not TGF-β or IFN-γ (Fig. 5b, c). Somewhat surprisingly, CD4+ CD25low/− cells expressed similar levels of IL-10 as CD4+ CD25high cells, and 3- to 6-fold-higher amounts of TGF-β (Fig. 5a, b). As expected, CD4+ CD25low/− cells also expressed high levels of IFN-γ (20- and 28-fold difference versus CD25high cells) (Fig. 5c), demonstrating a mixed phenotype in CD4+ CD25low/− cells, expressing both suppressive and proinflammatory cytokines. The IL-10 relative to IFN-γ expression was more than 20 fold higher in CD4+ CD25high versus CD4+ CD25low/− cells (Fig. 5d), showing a more uniformly suppressive phenotype of CD4+ CD25high cells.
Analysis of mRNA of sorted T-cell populations. Isolated lamina propria lymphocytes from tumor and tumor-free gastric mucosa, and PBMC from gastric cancer patients or healthy controls, were sorted into CD25high (“CD25hi”) and CD25low/− (“CD25lo”) CD4 T cells and analyzed for expression of interleukin (IL)-10 (a), transforming growth factor (TGF)-β (b), or interferon (IFN)-γ (c) mRNA using RT-PCR. a–c Data (a–c) are expressed as 2−ΔCT, relative to the housekeeping gene HPRT. d Expression of IL-10 divided by IFN-γ. In the box plots, the horizontal line represents median, the box edges show the interquartile range (first and third quartile), and the whiskers depict maximum and minimum values, with the exception of outliers. Existing outliers (>1.5 × interquartile range) are shown as dots. For gastric cancer patients, n = 7 for tumor-free tissue and n = 5 for tumor tissue and blood; for healthy controls, n = 4; p values refer to Mann–Whitney tests
Discussion
An increase of regulatory CD4 T cells within different types of tumors has recently been reported in several studies [12, 36, 37]. It has been suggested that these cells help the tumor to modulate the antitumor immune response mainly by inhibiting T-cell-mediated tumor killing [38, 39]. The suppressive activity has been assigned to the partially overlapping CD4+ CD25high [40] as well as the CD4+ FOXP3+ T-cell populations [22, 33]. There is also evidence of tumor antigen-specific Tregs [41]. Although expression of FOXP3 has been linked with suppressive activity, it is not clear whether it is sufficient with FOXP3 expression to confer a Treg phenotype, because ectopic FOXP3 expression does not always lead to development of suppressive function [42]. However, FOXP3 expression seems to be naturally induced in peripheral tissue, and this leads to acquisition of Treg activity [18].
We have previously shown that stomach mucosal CD4+ CD25high T cells have a suppressive function affecting both proliferation and IFN-γ production of CD4+ CD25− T cells [22]. In this study we have further analyzed the CD4+ CD25high population by analyzing the gene transcription of IL-10, IFN-γ, and TGF-β in sorted cells. The CD4+ CD25high cells were found to have a higher IL-10/IFN-γ expression ratio compared to CD4+ CD25low/− T cells, indicating a uniformly suppressive phenotype of these cells. TGF-β is known to be an important cytokine for the regulatory T cells [43, 44], but in our analysis, CD4+ CD25low/− T cells in the mucosa expressed higher transcription levels of TGF-β compared to CD4+ CD25high. In line with this, it has been shown that the suppressive function of Treg cells from TGF-β1-deficient mice was abrogated by anti-TGF-β monoclonal antibodies, indicating that functional TGF-β can be provided by a non-Treg cell source [45]. This result agrees with our findings: we show that CD4+ CD25high T cells are not the main producers of TGF-β in gastric tissue, and that CD4+ CD25low/− T cells have a mixed phenotype capable of producing both suppressive and inflammatory cytokines. IL-10 production by Treg and other cells is most likely beneficial for the tumor and detrimental to the patient. Previously, we have shown that both blood and gastric T cells from gastric cancer patients produce high levels of IL-10 after in vitro stimulation [46]. Furthermore, in a recent study it was shown that IL-10 production by gastric cancer cells correlates with dramatically reduced survival [47].
The increase of Tregs within tumors may be caused by several factors modulated by the tumors. Not only is the recruitment of Tregs increased [22, 23], but induction of FOXP3 expression at the mucosal site may be promoted by IFN-γ as well as other factors. For instance, gastric cancers express high levels of the enzyme COX-2 [48, 49], which mediates production of prostaglandin E2 (PGE2). Treatment of CD4+ CD25− T cells with PGE2 has been shown to upregulate FOXP3 and cause a suppressive function [50]; this mechanism has also been shown in colon cancer [41] and lung cancer [51].
In addition to active recruitment or induced expression of FOXP3 as an explanation for increased Treg numbers, we investigated a third possibility in this study, and determined the proliferation of CD4+ FOXP3+ T cells. Our data show that there is a significantly higher proportion of Ki67+ among CD4+ FOXP3+ compared to CD4+ FOXP3− in gastric tumor and nontumor tissue as well as in peripheral blood. Although high proliferation of CD4+ FOXP3+ T cells may contribute to high frequencies of Treg in the mucosa, our data do not support that proliferation is the major explanation for higher Treg numbers in tumor compared to nontumor tissue, because FOXP3+ proliferation is equally increased in both these locations.
The finding of high proliferation among peripheral blood FOXP3+ T cells is supported by another study demonstrating that Treg from peripheral blood have lower levels of T-cell-receptor excision circles, which indicated that the cells have undergone many cell divisions [52]. It has also been shown that peripheral blood CD4+ FOXP3+ T cells have a higher expression of the proliferation marker Ki67 [53]. Importantly, we show that proliferating CD4+ FOXP3+ T cells exhibit a complete demethylation of the analyzed CpG site in the FOXP3 promoter, which indicates that these cells have a stable Treg phenotype and are thus not activated effector T cells with a transient FOXP3 expression profile [33]. Our demethylation data are limited by the fact that we could not analyze tissue-derived T cells. However, a study of colon tissue indicates that methylation patterns of FOXP3+ cells in tissue and blood are comparable [54], which suggests that our results are valid for stomach Treg.
We and others have shown that gastric cancer patients have higher numbers of Treg not only in tissue but also in the blood compared to healthy controls [21, 36], and gastric cancer patients from whom tumors have been removed have significantly lower levels of CD4+ CD25high T cells [12]. These data are supported by Cao et al., who concluded that hepatic cancer cells cultured together with PBMC promote CD4+ CD25high T-cell proliferation and inhibit CD4+ CD25− T-cell proliferation [24]. However, our data did not establish an altered proliferation of peripheral blood CD4+ CD25high T cells of cancer patients compared to healthy controls.
In summary, we show a high degree of proliferation among regulatory CD4+ FOXP3+ CD25high T cells in the gastric mucosa both in the tumor and in the surrounding tissue. These local Treg were not induced in the tissue and expressed a suppressive cytokine profile characterized by high IL-10 and low TGF-β and IFN-γ production.
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108
Brenner H, Rothenbacher D, Arndt V. Epidemiology of stomach cancer. Methods Mol Biol. 2009;472:467–77.
Atherton JC. The pathogenesis of Helicobacter pylori-induced gastro-duodenal diseases. Annu Rev Pathol. 2006;1:63–96.
Mueller D, Tegtmeyer N, Brandt S, Yamaoka Y, Poire ED, Sgouras D, et al. c-Src and c-Abl kinases control hierarchic phosphorylation and function of the CagA effector protein in Western and East Asian Helicobacter pylori strains. J Clin Invest. 2012;122:1553–66.
Tsugawa H, Suzuki H, Saya H, Hatakeyama M, Hirayama T, Hirata K, et al. Reactive oxygen species-induced autophagic degradation of Helicobacter pylori CagA is specifically suppressed in cancer stem-like cells. Cell Host Microbe. 2012;12:764–77.
Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70.
Conroy H, Marshall NA, Mills KHG. TLR ligand suppression or enhancement of Treg cells? A double-edged sword in immunity to tumours. Oncogene. 2008;27:168–80.
Grauer OM, Nierkens S, Bennink E, Toonen LWJ, Boon L, Wesseling P, et al. CD4+ FoxP3+ regulatory T cells gradually accumulate in gliomas during tumor growth and efficiently suppress antiglioma immune responses in vivo. Int J Cancer. 2007;121:95–105.
Saeger H-D, Klöppel G, Kabelitz D, Kalthoff H, Hinz S, Pagerols-Raluy L, et al. Foxp3 expression in pancreatic carcinoma cells as a novel mechanism of immune evasion in cancer. Cancer Res. 2007;67:8344–50.
Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9.
Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, et al. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766–72.
Kono K, Kawaida H, Takahashi A, Sugai H, Mimura K, Miyagawa N, et al. CD4(+)CD25high regulatory T cells increase with tumor stage in patients with gastric and esophageal cancers. Cancer Immunol Immunother. 2006;55:1064–71.
Yoshii M, Tanaka H, Ohira M, Muguruma K, Iwauchi T, Lee T, et al. Expression of Forkhead box P3 in tumour cells causes immunoregulatory function of signet ring cell carcinoma of the stomach. Br J Cancer. 2012;106:1668–74.
Zhou S, Xu S, Tao H, Zhen Z, Chen G, Zhang Z, et al. CCR7 Expression and Intratumoral FOXP3+ Regulatory T-cells are correlated with overall survival and lymph node metastasis in gastric cancer. PLoS One [Internet]. 2013 [cited 2015 Feb 5];8. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3764061/.
Fujii S, Sakaguchi S, Yagi H, Nomura T, Nakamura K, Yamazaki S, et al. Crucial role of FOXP3 in the development and function of human CD25+ CD4+ regulatory T-cells. Int Immunol. 2004;16:1643–56.
Hori S, Nomura T, Sakaguchi S. Control of regulatory T-cell development by the transcription factor Foxp3. Science. 2003;299:1057–61.
Rudensky AY, Gavin MA, Torgerson TR, Houston E, DeRoos P, Ho WY, et al. Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T-cell development. Proc Natl Acad Sci USA. 2006;103:6659–64.
Walker MR, Kasprowicz DJ, Gersuk VH, Benard A, Van Landeghen M, Buckner JH, et al. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+ CD25− T cells. J. Clin. Invest. 2003;112:1437–43.
Pillai V, Ortega SB, Wang CK, Karandikar NJ. Transient regulatory T-cells: a state attained by all activated human T-cells. Clin Immunol. 2007;123:18–29.
Lundgren A, Strömberg E, Sjöling A, Lindholm C, Enarsson K, Edebo A, et al. Mucosal FOXP3-expressing CD4+ CD25high regulatory T-cells in Helicobacter pylori-infected patients. Infect Immun. 2005;73:523–31.
Enarsson K, Lundin BS, Johnsson E, Brezicka T, Quiding-Järbrink M. CD4+ CD25high regulatory T-cells reduce T-cell transendothelial migration in cancer patients. Eur J Immunol. 2007;37:282–91.
Enarsson K, Lundgren A, Kindlund B, Hermansson M, Roncador G, Banham AH, et al. Function and recruitment of mucosal regulatory T-cells in human chronic Helicobacter pylori infection and gastric adenocarcinoma. Clin Immunol. 2006;121:358–68.
Mizukami Y, Kono K, Kawaguchi Y, Akaike H, Kamimura K, Sugai H, et al. CCL17 and CCL22 chemokines within tumor microenvironment are related to accumulation of Foxp3+ regulatory T-cells in gastric cancer. Int J Cancer. 2008;122:2286–93.
Cao M, Cabrera R, Xu Y, Firpi R, Zhu H, Liu C, et al. Hepatocellular carcinoma cell supernatants increase expansion and function of CD4(+)CD25(+) regulatory T-cells. Lab Invest. 2007;87:582–90.
Forman D. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Helicobacter and Cancer Collaborative Group. Gut 2001;49:347–353
Janzon A, Sjöling A, Lothigius A, Ahmed D, Qadri F, Svennerholm A-M. Failure to detect Helicobacter pylori DNA in drinking and environmental water in Dhaka, Bangladesh, using highly sensitive real-time PCR assays. Appl Environ Microbiol. 2009;75:3039–44.
Mattsson A, Tinnert A, Hamlet A, Lönroth H, Bölin I, Svennerholm AM. Specific antibodies in sera and gastric aspirates of symptomatic and asymptomatic Helicobacter pylori-infected subjects. Clin Diagn Lab Immunol. 1998;5:288–93.
Lundgren A, Suri-Payer E, Enarsson K, Svennerholm A-M, Lundin BS. Helicobacter pylori-specific CD4+ CD25high regulatory T-cells suppress memory T-cell responses to H. pylori in infected individuals. Infect Immun. 2003;71:1755–62.
Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+ FOXP3 T-cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood. 2007;110:2983–90.
Kindlund B, Sjöling A, Hansson M, Edebo A, Hansson L-E, Sjövall H, et al. FOXP3-expressing CD4(+) T-cell numbers increase in areas of duodenal gastric metaplasia and are associated to CD4(+) T-cell aggregates in the duodenum of Helicobacter pylori-infected duodenal ulcer patients. Helicobacter. 2009;14:192–201.
Bustin SA. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and problems. J Mol Endocrinol. 2002;29:23–39.
Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39:782–95.
Janson PCJ, Winerdal ME, Marits P, Thörn M, Ohlsson R, Winqvist O. FOXP3 promoter demethylation reveals the committed Treg population in humans. PLoS One. 2008;3:e1612.
Wang J, Ioan-Facsinay A, van der Voort EIH, Huizinga TWJ, Toes REM. Transient expression of FOXP3 in human activated nonregulatory CD4+ T-cells. Eur J Immunol. 2007;37:129–38.
Schmitt E, Klein-Hessling S, Serfling E, Hamann A, Huehn J, Floess S, et al. Epigenetic control of the foxp3 locus in regulatory T-cells. PLoS Biol. 2007;5:e38.
Ichihara F, Kono K, Takahashi A, Kawaida H, Sugai H, Fujii H. Increased populations of regulatory T-cells in peripheral blood and tumor-infiltrating lymphocytes in patients with gastric and esophageal cancers. Clin Cancer Res. 2003;9:4404–8.
Goedegebuure PS, Linehan DC, Liyanage UK, Moore TT, Joo H-G, Tanaka Y, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J. Immunol. 2002;169:2756–61.
Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–8.
Reiman JM, Kmieciak M, Manjili MH, Knutson KL. Tumor immunoediting and immunosculpting pathways to cancer progression. Semin Cancer Biol. 2007;17:275–87.
Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+ CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–53.
Yaqub S, Henjum K, Mahic M, Jahnsen FL, Aandahl EM, Bjørnbeth BA, et al. Regulatory T-cells in colorectal cancer patients suppress anti-tumor immune activity in a COX-2 dependent manner. Cancer Immunol Immunother. 2008;57:813–21.
Allan SE, Passerini L, Bacchetta R, Crellin N, Dai M, Orban PC, et al. The role of 2 FOXP3 isoforms in the generation of human CD4+ Tregs. J. Clin. Invest. 2005;115:3276–84.
Zheng SG, Wang JH, Gray JD, Soucier H, Horwitz DA. Natural and induced CD4+ CD25+ cells educate CD4+CD25− cells to develop suppressive activity: the role of IL-2, TGF-beta, and IL-10. J Immunol. 2004;172:5213–21.
Nakamura K, Kitani A, Fuss I, Pedersen A, Harada N, Nawata H, et al. TGF-beta 1 plays an important role in the mechanism of CD4+ CD25+ regulatory T-cell activity in both humans and mice. J Immunol. 2004;172:834–42.
Fahlén L, Read S, Gorelik L, Hurst SD, Coffman RL, Flavell RA, et al. T-cells that cannot respond to TGF-beta escape control by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2005;201:737–46.
Lundin BS, Enarsson K, Kindlund B, Lundgren A, Johnsson E, Quiding-Järbrink M, et al. The local and systemic T-cell response to Helicobacter pylori in gastric cancer patients is characterised by production of interleukin-10. Clin Immunol. 2007;125:205–13.
Sakamoto T, Saito H, Tatebe S, Tsujitani S, Ozaki M, Ito H, et al. Interleukin-10 expression significantly correlates with minor CD8+ T-cell infiltration and high microvessel density in patients with gastric cancer. Int J Cancer. 2006;118:1909–14.
Hatakeyama M, Brzozowski T. Pathogenesis of Helicobacter pylori infection. Helicobacter. 2006;11(suppl 1):14–20.
Ribeiro ML, Bartchewsky W, Martini MR, Masiero M, Squassoni AC, Alvarez MC, et al. Effect of Helicobacter pylori infection on IL-8, IL-1beta and COX-2 expression in patients with chronic gastritis and gastric cancer. Scand J Gastroenterol. 2009;44:153–61.
Dubinett SM, Baratelli F, Lin Y, Zhu L, Yang S-C, Heuzé-Vourc′h N, et al. Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J Immunol. 2005;175:1483–90.
Sharma S, Yang S-C, Zhu L, Reckamp K, Gardner B, Baratelli F, et al. Tumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer Res. 2005;65:5211–20.
Wolf D, Rumpold H, Koppelstätter C, Gastl GA, Steurer M, Mayer G, et al. Telomere length of in vivo expanded CD4(+)CD25 (+) regulatory T cells is preserved in cancer patients. Cancer Immunol Immunother. 2006;55:1198–208.
Tuovinen H, Laurinolli T-T, Rossi LH, Pekkarinen PT, Mattila I, Arstila TP. Thymic production of human FOXP3(+) regulatory T-cells is stable but does not correlate with peripheral FOXP3 expression. Immunol Lett. 2008;117:146–53.
Wieczorek G, Asemissen A, Model F, Turbachova I, Floess S, Liebenberg V, et al. Quantitative DNA methylation analysis of FOXP3 as a new method for counting regulatory T cells in peripheral blood and solid tissue. Cancer Res. 2009;69:599–608.
Acknowledgments
We are grateful to the patients participating in this study, and to the staff at Dept. of Surgery, Sahlgrenska University Hospital. Especially acknowledged are Magdalena Granung and Junette Ohlin for valuable help with patient recruitment and Lisbeth Eklund for patient information. This study was supported by grants from Assar Gabrielsson’s cancer foundation, the Swedish Cancer Society, the Swedish Research Council, and LUA-ALF-Göteborg. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent or substitute for it was obtained from all patients for being included in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kindlund, B., Sjöling, Å., Yakkala, C. et al. CD4+ regulatory T cells in gastric cancer mucosa are proliferating and express high levels of IL-10 but little TGF-β. Gastric Cancer 20, 116–125 (2017). https://doi.org/10.1007/s10120-015-0591-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-015-0591-z