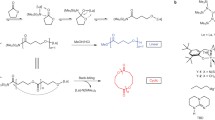
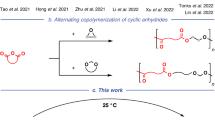
Abstract
Preparation of chemically recyclable polyesters by ring-opening polymerization (ROP) has made a considerable progress over the past few years. However, this method involves cumbersome synthesis and minimal functional diversity of cyclic monomers. Therefore, it is of great significance to develop novel polymerization methods for direct polymerization of commercially available monomers to prepare recyclable polyesters with versatile functionalities. In present work, we report dehydrogenative copolymerization of commercial α,ω-diols to afford high molecular weight chemically recyclable aliphatic copolyesters (65.7 kg·mol−1) by using commercially available Milstein catalyst precursor. The thermal properties of the obtained copolymers could be finely tuned by simply adjusting the feeding ratio of two monomers. The incorporation of aliphatic or aromatic rings into polyester mainchain via copolymerization of 1,10-decanediol with 1,4-cyclohexanedimethanol and 1,4-benzenedimethanol could significantly improve the thermal properties of the resulting copolymers. More importantly, the obtained copolyesters were able to completely depolymerize back to original diols via hydrogenation by the same catalyst in solvent-free and mild conditions, thus offering a green and cost-effective route toward the preparation of widely used polyesters.
Similar content being viewed by others
References
Geyer, R.; Jambeck, J. R.; Law, K. L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782.
Stubbins, A.; Law, K. L.; Muñoz, S. E.; Bianchi, T. S.; Zhu, L. Plastics in the Earth system. Science 2021, 373, 51–55.
Hillmyer, M. A.; Tolman, W. B. Aliphatic polyester block polymers: renewable, degradable, and sustainable. Acc. Chem. Res. 2014, 47, 2390–2396.
Korley, L. T. J.; Epps, T. H., III; Helms, B. A.; Ryan, A. J. Toward polymer upcycling-adding value and tackling circularity. Science 2021, 373, 66–69.
Kamber, N. E.; Jeong, W.; Waymouth, R. M.; Pratt, R. C.; Lohmeijer, B. G. G.; Hedrick, J. L. Organocatalytic ring-opening polymerization. Chem. Rev. 2007, 107, 5813–5840.
Feng, Z.; Wu, L.; Dong, H.; Liu, B.; Cheng, R. Copolyesters of ε-caprolactone and L-lactide catalyzed by a tetrabutylammonium phthalimide-N-oxyl organocatalyst. RSC Adv. 2021, 11, 19021–19028.
Tang, X.; Shi, C.; Zhang, Z.; Chen, E. Y. X. Toughening biodegradable isotactic poly(3-hydroxybutyrate) via stereoselective copolymerization of a diolide and lactones. Macromolecules 2021, 54, 9401–9409.
Tang, X.; Westlie, A. H.; Caporaso, L.; Cavallo, L.; Falivene, L.; Chen, E. Y. X. Biodegradable polyhydroxyalkanoates by stereoselective copolymerization of racemic diolides: stereocontrol and polyolefin-like properties. Angew. Chem. Int. Ed. 2020, 59, 7881–7890.
Tang, X.; Westlie, A. H.; Watson, E. M.; Chen, E. Y. X. Stereosequenced crystalline polyhydroxyalkanoates from diastereomeric monomer mixtures. Science 2019, 366, 754–758.
Kocen, A. L.; Cui, S. L.; Lin, T. W.; LaPointe, A. M.; Coates, G. W. Chemically recyclable ester-linked polypropylene. J. Am. Chem. Soc. 2022, 144, 12613–12618.
Häußler, M.; Eck, M.; Rothauer, D.; Mecking, S. Closed-loop recycling of polyethylene-like materials. Nature 2021, 590, 423–427.
Coates, G. W.; Getzler, Y. D. Y. L. Chemical recycling to monomer for an ideal, circular polymer economy. Nat. Mater. 2020, 5, 501–516.
Zhao, J. Z.; Yue, T. J.; Ren, B. H.; Liu, Y.; Ren, W. M.; Lu, X. B. Recyclable sulfur-rich polymers with enhanced thermal, mechanical, and optical performance. Macromolecules 2022, 10.1021/acs.macromol.2c01628.
Liao, X.; Cui, F. C.; He, J. H.; Ren, W. M.; Lu, X. B.; Zhang, Y. T. A sustainable approach for the synthesis of recyclable cyclic CO2-based polycarbonates. Chem. Sci. 2022, 13, 6283–6290.
Liu, Y.; Zhou, H.; Guo, J. Z.; Ren, W. M.; Lu, X. B. Completely recyclable monomers and polycarbonate: approach to sustainable polymers. Angew. Chem. Int. Ed. 2017, 56, 4862–4866.
Yuan, P.; Sun, Y.; Xu, X.; Luo, Y.; Hong, M. Towards high-performance sustainable polymers via isomerization-driven irreversible ring-opening polymerization of five-membered thionolactones. Nat. Chem. 2021, 14, 294–303.
Fan, H. Z.; Yang, X.; Chen, J. H.; Tu, Y. M.; Cai, Z.; Zhu, J. B. Advancing the development of recyclable aromatic polyesters by functionalization and stereocomplexation. Angew. Chem. Int. Ed. 2022, 61, e202117639.
Tu, Y. M.; Wang, X. M.; Yang, X.; Fan, H. Z.; Gong, F. L.; Cai, Z.; Zhu, J. B. Biobased high-performance aromatic-aliphatic polyesters with complete recyclability. J. Am. Chem. Soc. 2021, 143, 20591–20597.
Zhu, J. B.; Watson, E. M.; Tang, J.; Chen, E. Y. X. A synthetic polymer system with repeatable chemical recyclability. Science 2018, 360, 398–403.
Hong, M.; Chen, E. Y. X. Completely recyclable biopolymers with linear and cyclic topologies via ring-opening polymerization of γ-butyrolactone. Nat. Chem. 2016, 8, 42–49.
Zhu, J. B.; Chen, E. Y. X. Living coordination polymerization of a six-five bicyclic lactone to produce completely recyclable polyester. Angew. Chem. Int. Ed. 2018, 57, 12558–12562.
Zhu, J. B.; Chen, E. Y. X. Catalyst-sidearm-induced stereoselectivity switching in polymerization of a racemic lactone for stereocomplexed crystalline polymer with a circular life cycle. Angew. Chem. Int. Ed. 2019, 58, 1178–1182.
Tang, X.; Hong, M.; Falivene, L.; Caporaso, L.; Cavallo, L.; Chen, E. Y. X The quest for converting biorenewable bifunctional α-methylene-γ-butyrolactone into degradable and recyclable polyester: controlling vinyl-addition/ring-opening/cross-linking pathways. J. Am. Chem. Soc. 2016, 138, 14326–14337.
Hong, M.; Chen, E. Y. X. Towards truly sustainable polymers: a metal-free recyclable polyester from biorenewable non-strained γ-butyrolactone. Angew. Chem. Int. Ed. 2016, 55, 4188–4193.
Hong, M.; Tang, X.; Newell, B. S.; Chen, E. Y. X. “Nonstrained” γ-butyrolactone-based copolyesters: copolymerization characteristics and composition-dependent (thermal, eutectic, cocrystallization, and degradation) properties. Macromolecules 2017, 50, 8469–8479.
Xiong, M. Y.; Schneiderman, D. K.; Bates, F. S.; Hillmyer, M. A.; Zhang, K. C. Scalable production of mechanically tunable block polymers from sugar. Proc. Natl. Acad. Sci. U.S.A 2014, 111, 8357–8362.
Fahnhorst, G. W.; Hoye, T. R. A Carbomethoxylated polyvalerolactone from malic acid: synthesis and divergent chemical recycling. ACS Macro. Lett. 2018, 7, 143–147.
Li, J.; Liu, F.; Liu, Y.; Shen, Y.; Li, Z. Functionalizable and chemically recyclable thermoplastics from chemoselective ring-opening polymerization of bio-renewable bifunctional α-methylene-δ-valerolactone. Angew. Chem. Int. Ed. 2022, 61, e202207105.
Li, C.; Wang, L.; Yan, Q.; Liu, F.; Shen, Y.; Li, Z. Rapid and controlled polymerization of bio-sourced δ-caprolactone toward fully recyclable polyesters and thermoplastic elastomers. Angew. Chem. Int. Ed. 2022, 61, e202201407.
Zhang, J.; Leitus, G.; Ben-David, Y.; Milstein, D. Facile conversion of alcohols into esters and dihydrogen catalyzed by new ruthenium complexes. J. Am. Chem. Soc. 2005, 127, 10840–10841.
Ben-Ari, E.; Leitus, G.; Shimon, L. J.; Milstein, D. Metal-ligand cooperation in C−H and H2 activation by an electron-rich PNP Ir(I) system: facile ligand dearomatization-aromatization as key steps. J. Am. Chem. Soc. 2006, 128, 15390–15391.
Gunanathan, C.; Ben-David, Y.; Milstein, D. Direct synthesis of amides from alcohols and amines with liberation of H2. Science 2007, 317, 790–792.
Zeng, H.; Guan, Z. Direct synthesis of polyamides via catalytic dehydrogenation of diols and diamines. J. Am. Chem. Soc. 2011, 133, 1159–1161.
Gnanaprakasam, B.; Balaraman, E.; Gunanathan, C.; Milstein, D. Synthesis of polyamides from diols and diamines with liberation of H2. J. Polym. Sci., Part A: Polym. Chem. 2012, 50, 1755–1765.
Hunsicker, D. M.; Dauphinais, B. C.; Mc Ilrath, S. P.; Robertson, N. J. Synthesis of high molecular weight polyesters via in vacuo dehydrogenation polymerization of diols. Macromol. Rapid Commun. 2012, 33, 232–236.
Zhang, J.; Leitus, G.; Ben-David, Y.; Milstein, D. Efficient homogeneous catalytic hydrogenation of esters to alcohols. Angew. Chem. Int. Ed. 2006, 45, 1113–1115.
Balaraman, E.; Gnanaprakasam, B.; Shimon, L. J.; Milstein, D. Direct hydrogenation of amides to alcohols and amines under mild conditions. J. Am. Chem. Soc. 2010, 132, 16756–16758.
Krall, E. M.; Klein, T. W.; Andersen, R. J.; Nett, A. J.; Glasgow, R. W.; Reader, D. S.; Dauphinais, B. C.; Mc Ilrath, S. P.; Fischer, A. A.; Carney, M. J.; Hudson, D. J.; Robertson, N. J. Controlled hydrogenative depolymerization of polyesters and polycarbonates catalyzed by ruthenium(II) PNN pincer complexes. Chem. Commun. 2014, 50, 4884–4887.
Kumar, A.; von Wolff, N.; Rauch, M.; Zou, Y. Q.; Shmul, G.; Ben-David, Y.; Leitus, G.; Avram, L.; Milstein, D. Hydrogenative depolymerization of nylons. J. Am. Chem. Soc. 2020, 142, 14267–14275.
Tang, X.; Chen, E. Y. X. Toward infinitely recyclable plastics derived from renewable cyclic esters. Chem 2019, 5, 284–312.
Gusev, D. G. Dehydrogenative coupling of ethanol and ester hydrogenation catalyzed by pincer-type YNP complexes. ACS Catal. 2016, 6, 6967–6981.
Gusev, D. G. Rethinking the dehydrogenative amide synthesis. ACS Catal. 2017, 7, 6656–6662.
Morris, S. A.; Gusev, D. G. Rethinking the Claisen-Tishchenko reaction. Angew. Chem. Int. Ed. 2017, 56, 6228–6231.
Nguyen, D. H.; Trivelli, X.; Capet, F.; Swesi, Y.; Favre-Réguillon, A.; Vanoye, L.; Dumeignil, F.; Gauvin, R. M. Deeper mechanistic insight into Ru Pincer-mediated acceptorless dehydrogenative coupling of alcohols: exchanges, intermediates, and deactivation species. ACS Catal. 2018, 8, 4719–4734.
Gusev, D. G. Revised mechanisms of the catalytic alcohol dehydrogenation and ester reduction with the Milstein PNN complex of ruthenium. Organometallics 2020, 39, 258–270.
Li, H.; Hall, M. B. Computational mechanistic studies on reactions of transition metal complexes with noninnocent pincer ligands: aromatization-dearomatization or not. ACS Catal. 2015, 5, 1895–1913.
Cho, D.; Ko, K. C.; Lee, J. Y. Catalytic mechanism for the ruthenium-complex-catalyzed synthesis of amides from alcohols and amines: a DFT study. Organometallics 2013, 32, 4571–4576.
Li, H.; Wang, X.; Wen, M.; Wang, Z. X. Computational insight into the mechanism of selective imine formation from alcohol and amine catalyzed by the ruthenium(II)-PNP Pincer complex. Eur. J. Inor. Chem. 2012, 2012, 5011–5020.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Nos. 22061027 and 22261034).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Conflict of Interests
The authors declare no interest conflict.
Electronic Supplementary Information
Rights and permissions
About this article
Cite this article
Xu, WM., Yu, YD., Ma, MX. et al. Green Synthesis of Chemically Recyclable Polyesters via Dehydrogenative Copolymerization of Diols. Chin J Polym Sci 41, 1206–1214 (2023). https://doi.org/10.1007/s10118-023-2903-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10118-023-2903-9