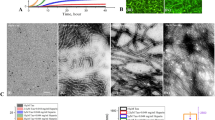
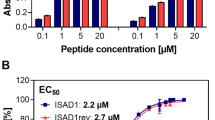
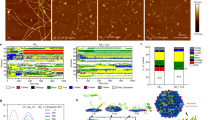
Abstract
After repeated frustrations with amyloid beta (Aβ)-targeted clinical trials for Alzheimer’s disease (AD) in recent years, the therapeutic focus of AD has gradually shifted from Aβ to tau protein. The misfolding and aggregation of tau protein into neurofibrillary tangles (NFTs) cause neuron death and synaptic dysfunction, and the deposition of NFTs is more closely related to the severity of AD than Aβ plaques. Thus, it has great potential to target tau protein aggregation for AD treatment. The hexapeptide VQIVYK (known as PHF6) in tau protein has been found to play a dominant role for tau aggregation and was widely used as a model to design tau protein aggregation inhibitors. Here, inspired by natural heat shock protein (HSPs), we fabricated a self-assembly nanochaperone based on mixed-shell polymeric micelle (MSPM) as a novel tau-targeted AD therapy. With tunable phase-separated microdomains on the surface, the nanochaperone could effectively bind with PHF6 aggregates, inhibit PHF6 aggregation, block neuronal internalization of PHF6 species, thus significantly alleviating PHF6 mediated neurotoxicity. Moreover, the as-prepared nanochaperone could work with proteinase to facilitate the degradation of PHF6 aggregates. This bioinspired nanochaperone demonstrated a new way to target tau protein and provided a promising strategy for AD treatment.
Similar content being viewed by others
References
Qian, X.; Hamad, B.; Dias-Lalcaca, G. The Alzheimer disease market. Nat. Rev. Drug Discovery 2015, 14, 675–676.
Du, Z.; Li, M.; Ren, J.; Qu, X. Current strategies for modulating Aβ aggregation with multifunctional agents. Acc. Chem. Res. 2021, 54, 2172–2184.
Ke, P. C.; Pilkington, E. H.; Sun, Y.; Javed, I.; Kakinen, A.; Peng, G.; Ding, F.; Davis, T. P. Mitigation of amyloidosis with nanomaterials. Adv. Mater. 2020, 32, 1901690.
Benek, O.; Korabecny, J.; Soukup, O. A Perspective on multi-target drugs for Alzheimer’s disease. Trends Pharmacol. Sci. 2020, 41, 434–445.
Dubois, B.; Feldman, H. H.; Jacova, C.; Hampel, H.; Molinuevo, J. L.; Blennow, K.; Dekosky, S. T.; Gauthier, S.; Selkoe, D.; Bateman, R.; Cappa, S.; Crutch, S.; Engelborghs, S.; Frisoni, G. B.; Fox, N. C.; Galasko, D.; Habert, M. O.; Jicha, G. A.; Nordberg, A.; Pasquier, F.; Rabinovici, G.; Robert, P.; Rowe, C.; Salloway, S.; Sarazin, M.; Epelbaum, S.; de Souza, L. C.; Vellas, B.; Visser, P. J.; Schneider, L.; Stern, Y.; Scheltens, P.; Cummings, J. L. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 2014, 13, 614–629.
Busche, M. A.; Hyman, B. T. Synergy between amyloid-β and tau in Alzheimer’s disease. Nat. Neurosci. 2020, 23, 1183–1193.
Busche, M. A.; Wegmann, S.; Dujardin, S.; Commins, C.; Schiantarelli, J.; Klickstein, N.; Kamath, T. V.; Carlson, G. A.; Nelken, I.; Hyman, B. T. Tau impairs neural circuits, dominating amyloid-β effects, in Alzheimer models in vivo. Nat. Neurosci. 2019, 22, 57–64.
Doody, R. S.; Raman, R.; Farlow, M.; Iwatsubo, T.; Vellas, B.; Joffe, S.; Kieburtz, K.; He, F.; Sun, X.; Thomas, R. G.; Aisen, P. S.; Alzheimer’s Disease Cooperative Study Steering, C.; Siemers, E.; Sethuraman, G.; Mohs, R.; Semagacestat Study, G. A phase 3 trial of semagacestat for treatment of Alzheimer’s disease. New Engl. J. Med. 2013, 369, 341–350.
Karran, E.; Hardy, J. A Critique of the Drug Discovery and Phase 3 Clinical programs targeting the amyloid hypothesis for Alzheimer disease. Ann. Neurol. 2014, 76, 185–205.
Relkin, N. R.; Thomas, R. G.; Rissman, R. A.; Brewer, J. B.; Rafii, M. S.; van Dyck, C. H.; Jack, C. R.; Sano, M.; Knopman, D. S.; Raman, R.; Szabo, P.; Gelmont, D. M.; Fritsch, S.; Aisen, P. S. A phase 3 trial of IV immunoglobulin for Alzheimer disease. Neurology 2017, 88, 1768–1775.
Giacobini, E.; Gold, G. Alzheimer disease therapy—moving from amyloid-beta to tau. Nat. Rev. Neurol. 2013, 9, 677–686.
Congdon, E. E.; Sigurdsson, E. M. Tau-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 2018, 14, 399–415.
Chen, L.; Du, Y.; Zhang, K.; Liang, Z.; Li, J.; Yu, H.; Ren, R.; Feng, J.; Jin, Z.; Li, F.; Sun, J.; Zhou, M.; He, Q.; Sun, X.; Zhang, H.; Tian, M.; Ling, D. Tau-targeted multifunctional nanocomposite for combinational therapy of Alzheimer’s disease. ACS Nano 2018, 12, 1321–1338.
Wang, Y.; Mandelkow, E. Tau in physiology and pathology. Nat. Rev. Neurosci. 2016, 17, 5–21.
Li, C.; Gotz, J. Tau-based therapies in neurodegeneration: opportunities and challenges. Nat. Rev. Drug Discovery 2017, 16, 863–883.
Bejanin, A.; Schonhaut, D. R.; La Joie, R.; Kramer, J. H.; Baker, S. L.; Sosa, N.; Ayakta, N.; Cantwell, A.; Janabi, M.; Lauriola, M.; O’Neil, J. P.; Gorno-Tempini, M. L.; Miller, Z. A.; Rosen, H. J.; Miller, B. L.; Jagust, W. J.; Rabinovici, G. D. Tau pathology and neurodegeneration contribute to cognitive impairment in Alzheimer’s disease. Brain 2017, 140, 3286–3300.
Al Mamun, A.; Uddin, M. S.; Mathew, B.; Ashraf, G. M. Toxic tau: structural origins of tau aggregation in Alzheimer’s disease. Neural Regen. Res. 2020, 15, 1417–1420.
Jouanne, M.; Rault, S.; Voisin-Chiret, A. S. Tau protein aggregation in Alzheimer’s disease: An attractive target for the development of novel therapeutic agents. Eur. J. Med. Chem. 2017, 139, 153–167.
Xu, L.; Ding, Y.; Ma, F.; Chen, Y.; Chen, G.; Zhu, L.; Long, J.; Ma, R.; Liu, Y.; Liu, J.; Huang, F.; Shi, L. Engineering a pathological tautargeted nanochaperone for selective and synergetic inhibition of tau pathology in Alzheimer’s disease. Nano Today 2022, 43, 101388.
Zhu, L.; Xu, L.; Wu, X.; Deng, F.; Ma, R.; Liu, Y.; Huang, F.; Shi, L. Tau-targeted multifunctional nanoinhibitor for Alzheimer’s disease. ACS Appl. Mater. Interfaces 2021, 13, 23328–23338.
Seidler, P. M.; Boyer, D. R.; Rodriguez, J. A.; Sawaya, M. R.; Cascio, D.; Murray, K.; Gonen, T.; Eisenberg, D. S. Structure-based inhibitors of tau aggregation. Nat. Chem. 2018, 10, 170–176.
Wischik, C. M.; Harrington, C. R.; Storey, J. M. D. Tau-aggregation inhibitor therapy for Alzheimer’s disease. Biochem. Pharmacol. 2014, 88, 529–539.
Ryan, P.; Patel, B.; Makwana, V.; Jadhav, H. R.; Kiefel, M.; Davey, A.; Reekie, T. A.; Rudrawar, S.; Kassiou, M. Peptides, peptidomimetics, and carbohydrate-peptide conjugates as amyloidogenic aggregation inhibitors for Alzheimer’s disease. ACS Chem. Neurosci. 2018, 9, 1530–1551.
Bittar, A.; Bhatt, N.; Kayed, R. Advances and considerations in AD tau-targeted immunotherapy. Neurobiol. Dis. 2020, 134, 104707.
Kim, Y. E.; Hipp, M. S.; Bracher, A.; Hayer-Hartl, M.; Ulrich Hartl, F. Molecular chaperone functions in protein folding and proteostasis. Annu. Rev. Biochem. 2013, 82, 323–355.
Balchin, D.; Hayer-Hartl, M.; Hartl, F. U. In vivo aspects of protein folding and quality control. Science 2016, 353, 7.
Freilich, R.; Arhar, T.; Abrams, J. L.; Gestwicki, J. E. Protein-protein interactions in the molecular chaperone network. Acc. Chem. Res. 2018, 51, 940–949.
Huang, C.; Rossi, P.; Saio, T.; Kalodimos, C. G. Structural basis for the antifolding activity of a molecular chaperone. Nature 2016, 537, 202–206.
Gorantla, N. V.; Chinnathambi, S. Tau protein squired by molecular chaperones during Alzheimer’s disease. J. Mol. Neurosci. 2018, 66, 356–368.
Pratt, W. B.; Gestwicki, J. E.; Osawa, Y.; Lieberman, A. P. Targeting Hsp90/Hsp70-based protein quality control for treatment of adult onset neurodegenerative diseases. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 353–371.
Mok, S.-A.; Condello, C.; Freilich, R.; Gillies, A.; Arhar, T.; Oroz, J.; Kadavath, H.; Julien, O.; Assimon, V. A.; Rauch, J. N.; Dunyak, B. M.; Lee, J.; Tsai, F. T. F.; Wilson, M. R.; Zweckstetter, M.; Dickey, C. A.; Gestwicki, J. E. Mapping interactions with the chaperone network reveals factors that protect against tau aggregation. Nat. Struct. Mol. Biol. 2018, 25, 384.
Kundel, F.; De, S.; Flagmeier, P.; Horrocks, M. H.; Kjaergaard, M.; Shammas, S. L.; Jackson, S. E.; Dobson, C. M.; Klenerman, D. Hsp70 inhibits the nucleation and elongation of tau and sequesters tau aggregates with high affinity. ACS Chem. Biol. 2018, 13, 636–646.
Baughman, H. E. R.; Pham, T. H. T.; Adams, C. S.; Nath, A.; Klevit, R. E. Release of a disordered domain enhances HspB1 chaperone activity toward tau. Proc. Natl. Acad. Sci. U.S.A. 2020, 117, 2923–2929.
Young, Z. T.; Mok, S. A.; Gestwicki, J. E. Therapeutic strategies for restoring tau homeostasis. Cold Spring Harb. Perspect Med. 2018, 8, a024612.
Von Bergen, M.; Friedhoff, P.; Biernat, J.; Heberle, J.; Mandelkow, E. M.; Mandelkow, E. Assembly of τ protein into Alzheimer paired helical filaments depends on a local sequence motif (306VQIVYK311) forming β structure. Proc. Natl. Acad. Sci. U.S.A. 2000, 97, 5129–5134.
Ganguly, P.; Do, T. D.; Larini, L.; Lapointe, N. E.; Sercel, A. J.; Shade, M. F.; Feinstein, S. C.; Bowers, M. T.; Shea, J. E. Tau assembly: the dominant role of PHF6 (VQIVYK) in microtubule binding region repeat R3. J. Phys. Chem. B 2015, 119, 4582–4593.
Chemerovski-Glikman, M.; Frenkel-Pinter, M.; Mdah, R.; Abu-Mokh, A.; Gazit, E.; Segal, D. Inhibition of the aggregation and toxicity of the minimal amyloidogenic fragment of tau by its prosubstituted analogues. Chem. Eur. J. 2017, 23, 9618–9624.
Belostozky, A.; Richman, M.; Lisniansky, E.; Tovchygrechko, A.; Chill, J. H.; Rahimipour, S. Inhibition of tau-derived hexapeptide aggregation and toxicity by a self-assembled cyclic d,l-alpha-peptide conformational inhibitor. Chem. Commun. 2018, 54, 5980–5983.
Fanni, A. M.; Vander Zanden, C. M.; Majewska, P. V.; Majewski, J.; Chi, E. Y. Membrane-mediated fibrillation and toxicity of the tau hexapeptide PHF6. J. Biol. Chem. 2019, 294, 15304–15317.
Sievers, S. A.; Karanicolas, J.; Chang, H. W.; Zhao, A.; Jiang, L.; Zirafi, O.; Stevens, J. T.; Munch, J.; Baker, D.; Eisenberg, D. Structure-based design of non-natural amino-acid inhibitors of amyloid fibril formation. Nature 2011, 475, 96–100.
Zheng, J.; Baghkhanian, A. M.; Nowick, J. S. A hydrophobic surface is essential to inhibit the aggregation of a tau-protein-derived hexapeptide. J. Am. Chem. Soc. 2013, 135, 6846–6852.
Zhang, X. C.; Zhang, X. Y.; Zhong, M. L.; Zhao, P.; Guo, C.; Li, Y.; Wang, T.; Gao, H. L. Selection of a D-enantiomeric peptide specifically binding to PHF6 for inhibiting tau aggregation in transgenic mice. ACS Chem. Neurosci. 2020, 11, 4240–4253.
Huang, F.; Wang, J.; Qu, A.; Shen, L.; Liu, J.; Liu, J.; Zhang, Z.; An, Y.; Shi, L. Maintenance of amyloid β peptide homeostasis by artificial chaperones based on mixed-shell polymeric micelles. Angew. Chem. Int. Ed. 2014, 53, 8985–8990.
Huang, F.; Qu, A.; Yang, H.; Zhu, L.; Zhou, H.; Liu, J.; Long, J.; Shi, L. Self-assembly molecular chaperone to concurrently inhibit the production and aggregation of amyloid beta peptide associated with Alzheimer’s disease. ACS Macro Lett. 2018, 7, 983–989.
Zhang, X.; Wang, L. Y.; Xu, J. F.; Chen, D. Y.; Shi, L. Q.; Zhou, Y. F.; Shen, Z. H. Polymeric supramolecular systems: design, assembly and functions. Acta Polymerica Sinica (in Chinese) 2019, 50, 973–987.
Su, L. Z.; Liu, Y.; Li, Y. F.; An, Y. L.; Shi, L. Q. Responsive polymeric nanoparticles for biofilm-infection control. Chinese J. Polym. Sci. 2021, 39, 1376–1391.
Yang, H.; Li, X.; Zhu, L.; Wu, X.; Zhang, S.; Huang, F.; Feng, X.; Shi, L. Heat shock protein inspired nanochaperones restore amyloid-β homeostasis for preventative therapy of Alzheimer’s disease. Adv. Sci. 2019, 1901844.
Huang, F.; Shen, L.; Wang, J.; Qu, A.; Yang, H.; Zhang, Z.; An, Y.; Shi, L. Effect of the surface charge of artificial chaperones on the refolding of thermally denatured lysozymes. ACS Appl. Mater. Interfaces 2016, 8, 3669–3678.
Goux, W. J.; Kopplin, L.; Nguyen, A. D.; Leak, K.; Rutkofsky, M.; Shanmuganandam, V. D.; Sharma, D.; Inouye, H.; Kirschner, D. A. The formation of straight and twisted filaments from short tau peptides. J. Biol. Chem. 2004, 279, 26868–26875.
KrishnaKumar, V. G.; Paul, A.; Gazit, E.; Segal, D. Mechanistic insights into remodeled tau-derived PHF6 peptide fibrils by naphthoquinone-tryptophan hybrids. Sci. Rep. 2018, 8, 71.
Garcia-Chame, M. A.; Gutierrez-Sanz, O.; Ercan-Herbst, E.; Haustein, N.; Filipiak, M. S.; Ehrnhoefer, D. E.; Tarasov, A. A transistor-based label-free immunosensor for rapid detection of tau protein. Biosens. Bioelectron. 2020, 159, 112129.
Cho, N.-J.; Frank, C. W.; Kasemo, B.; Hook, F. Quartz crystal microbalance with dissipation monitoring of supported lipid bilayers on various substrates. Nat. Protoc. 2010, 5, 1096–1106.
Wang, J.; Yin, T.; Huang, F.; Song, Y.; An, Y.; Zhang, Z.; Shi, L. Artificial chaperones based on mixed shell polymeric micelles: insight into the mechanism of the interaction of the chaperone with substrate proteins using forster resonance energy transfer. ACS Appl. Mater. Interfaces 2015, 7, 10238–10249.
Li, C.; Liu, X.; Zhang, Y.; Lv, J.; Huang, F.; Wu, G.; Liu, Y.; Ma, R.; An, Y.; Shi, L. Nanochaperones mediated delivery of insulin. Nano Lett. 2020, 20, 1755–1765.
Li, X.; Cai, X.; Zhang, Z.; Ding, Y.; Ma, R.; Huang, F.; Liu, Y.; Liu, J.; Shi, L. Mimetic heat shock protein mediated immune process to enhance cancer immunotherapy. Nano Lett. 2020, 20, 4454–4463.
Liu, Y.; Du, J. J.; Yan, M.; Lau, M. Y.; Hu, J.; Han, H.; Yang, O. O.; Liang, S.; Wei, W.; Wang, H.; Li, J. M.; Zhu, X. Y.; Shi, L. Q.; Chen, W.; Ji, C.; Lu, Y. F. Biomimetic enzyme nanocomplexes and their use as antidotes and preventive measures for alcohol intoxication. Nat. Nanotechnol. 2013, 8, 187–192.
Selvin, P. R. The renaissance of fluorescence resonance energy transfer. Nat. Struct. Biol. 2000, 7, 730–734.
Goedert, M. The ordered assembly of tau is the gain-of-toxic function that causes human tauopathies. Alzheimers Dement. 2016, 12, 1040–1050.
Ghag, G.; Bhatt, N.; Cantu, D. V.; Guerrero-Munoz, M. J.; Ellsworth, A.; Sengupta, U.; Kayed, R. Soluble tau aggregates, not large fibrils, are the toxic species that display seeding and cross-seeding behavior. Protein Sci. 2018, 27, 1901–1909.
Frenkel-Pinter, M.; Richman, M.; Belostozky, A.; Abu-Mokh, A.; Gazit, E.; Rahimipour, S.; Segal, D. Selective inhibition of aggregation and toxicity of a tau-derived peptide using its glycosylated analogues. Chem. Eur. J. 2016, 22, 5945–5952.
Chesser, A. S.; Pritchard, S. M.; Johnson, G. V. W. Tau clearance mechanisms and their possible role in the pathogenesis of Alzheimer disease. Front. Neurol. 2013, 4, 122.
Whyte, L. S.; Lau, A. A.; Hemsley, K. M.; Hopwood, J. J.; Sargeant, T. J. Endo-lysosomal and autophagic dysfunction: a driving factor in Alzheimer’s disease. J. Neurochem. 2017, 140, 703–717.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Nos. 51933006 and 52073306), Young Elite Scientists Sponsorship Program by Tianjin (No. TJSQNTJ-2020-18), the Nonprofit Central Research Institute Fund of Chinese Academy of Medical Sciences (No. 2019-RC-HL-014) and Wenzhou Key Laboratory of Biomaterials and Engineering (No. WIUCASSWCL21004).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Notes
The authors declare no competing financial interest.
Electronic Supplementary Information (ESI)
Rights and permissions
About this article
Cite this article
Zhu, L., Zhang, MQ., Jing, HR. et al. Bioinspired Self-assembly Nanochaperone Inhibits Tau-Derived PHF6 Peptide Aggregation in Alzheimer’s Disease. Chin J Polym Sci 40, 1062–1070 (2022). https://doi.org/10.1007/s10118-022-2799-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10118-022-2799-9