Abstract
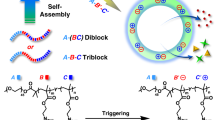
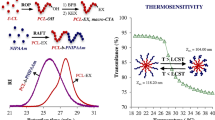
Programmed release of small molecular drugs from polymersomes is of great importance in drug delivery. A significant challenge is to adjust the membrane permeability in a well-controlled manner. Herein, we propose a strategy for controlling membrane phase separation by photo-cross-linking of the membrane-forming blocks with different molecular architectures. We synthesized three amphiphilic block copolymers with different membrane-forming blocks, which are poly(ethylene oxide)43-b-poly((ε-caprolactone)45-stat-((a-(cinnamoyloxymethyl)-1,2,3-triazol)caprolactone)25) (PEO43-b-P(CL45-stat-CTCL25)), PEO43-b-P(CL108-stat-CTCL16), and PEO43-b-PCTCL4-b-PCL79. These polymers were self-assembled into polymersomes using either a solvent-switch or powder rehydration method, and the obtained polymersomes were characterized by dynamic light scattering and transmission electron microscopy. Then the phase separation patterns within the polymersome membranes were investigated by mesoscopic dynamics (MesoDyn) simulations. To further confirm the change of the membrane permeability that resulted from the phase separation within the membrane, doxorubicin, as a small molecular drug, was loaded and released from the polymersomes. Due to the incompatibility between membrane-forming moieties (PCTCL and PCL), phase separation occurs and the release rate can be tuned by controlling the membrane phase pattern or by photo-cross-linking. Moreover, besides the compacting effect by formation of chemical bonds in the membrane, the cross-linking process can act as a driving force to facilitate the rearrangement and re-orientation of the phase pattern, which also influences the drug release behavior by modulating the cross-membrane distribution of the amorphous PCTCL moieties. In this way, the strategy of focusing on the membrane phase separation for the preparation of the polymersomes with finely tunable drug release rate can be envisioned and designed accordingly, which is of great significance in the field of delivery vehicles for programmed drug release.
Similar content being viewed by others
References
Tibbitt, M. W.; Dahlman, J. E.; Langer, R. Emerging frontiers in drug delivery. J. Am. Chem. Soc. 2016, 138, 704–717.
Dou, Y.; Li, C. W.; Li, L. L.; Guo, J. W.; Zhang, J. X. Bioresponsive drug delivery systems for the treatment of inflammatory diseases. J. Control. Release 2020, 327, 641–666.
Hassan, S.; Prakash, G.; Ozturk, A. B.; Saghazadeh, S.; Sohail, M. F.; Seo, J.; Dokmeci, M. R.; Zhang, Y. S.; Khademhosseini, A. Evolution and clinical translation of drug delivery nanomaterials. Nano Today 2017, 15, 91–106.
Prasanna, A.; Pooja, R.; Suchithra, V.; Ravikumar, A.; Gupta, P. K.; Niranjan, V. Smart drug delivery systems for cancer treatment using nanomaterials. Mater. Today: Proc. 2018, 5, 21047–21054.
Rasheed, T.; Nabeel, F.; Raza, A.; Bilal, M.; Iqbal, H. M. N. Biomimetic nanostructures/cues as drug delivery systems: a review. Mater. Today Chem. 2019, 13, 147–157.
Jiang, J.; Zhuravlev, E.; Hu, W. B.; Schick, C.; Zhou, D. S. The effect of self-nucleation on isothermal crystallization kinetics of poly(butylene succinate) (PBS) investigated by differential fast scanning calorimetry. Chinese J. Polym. Sci. 2017, 35, 1009–1019.
Sharma, A. K.; Prasher, P.; Aljabali, A. A.; Mishra, V.; Gandhi, H.; Kumar, S.; Mutalik, S.; Chellappan, D. K.; Tambuwala, M. M.; Dua, K.; Kapoor, D. N. Emerging era of “somes”: polymersomes as versatile drug delivery carrier for cancer diagnostics and therapy. Drug Deliv. Transl. Res. 2020, 10, 1171–1190.
Lee, J. S.; Feijen, J. Polymersomes for drug delivery: design, formation and characterization. J. Control. Release 2012, 161, 473–483.
Liu, D. Q.; Sun, H.; Xiao, Y. F.; Chen, S.; Cornel, E. J.; Zhu, Y. Q.; Du, J. Z. Design principles, synthesis and biomedical applications of polymer vesicles with inhomogeneous membranes. J. Control. Release 2020, 326, 365–386.
Fenton, O. S.; Olafson, K. N.; Pillai, P. S.; Mitchell, M. J.; Langer, R. Advances in biomaterials for drug delivery. Adv. Mater. 2018, 30, 1705328.
Allen, T. M.; Cullis, P. R. Drug delivery systems: Entering the mainstream. Science 2004, 303, 1818–1822.
Felice, B.; Prabhakaran, M. P.; Rodriguez, A. P.; Ramakrishna, S. Drug delivery vehicles on a nano-engineering perspective. Mater. Sci. Eng., C 2014, 41, 178–195.
Sun, R.; Qiu, N. S.; Shen, Y. Q. Polymeric cancer nanomedicines: Challenge and development. Acta Polymerica Sinica (in Chinese) 2019, 50, 588–601.
Davoodi, P.; Lee, L. Y.; Xu, Q. X.; Sunil, V.; Sun, Y. J.; Soh, S.; Wang, C. H. Drug delivery systems for programmed and on-demand release. Adv. Drug Deliv. Rev. 2018, 132, 104–138.
Uhrich, K. E.; Cannizzaro, S. M.; Langer, R. S.; Shakesheff, K. M. Polymeric systems for controlled drug release. Chem. Rev. 1999, 99, 3181–3198.
He, F.; Zhang, M. J.; Wang, W.; Cai, Q. W.; Su, Y. Y.; Liu, Z.; Faraj, Y.; Ju, X. J.; Xie, R.; Chu, L. Y. Designable polymeric microparticles from droplet microfluidics for controlled drug release. Adv. Mater. Technol. 2019, 4, 1800687.
Le Meins, J. F.; Sandre, O.; Lecommandoux, S. Recent trends in the tuning of polymersomes’ membrane properties. Eur. Phys. J. E 2011, 34, 14.
Chidanguro, T.; Ghimire, E.; Liu, C. H.; Simon, Y. C. Polymersomes: breaking the glass ceiling. Small 2018, 14, 1802734.
Du, F. F.; Bobbala, S.; Yi, S. J.; Scott, E. A. Sequential intracellular release of water-soluble cargos from shell-crosslinked polymersomes. J. Control. Release 2018, 282, 90–100.
Miller, A. J.; Pearce, A. K.; Foster, J. C.; O’Reilly, R. K. Probing and tuning the permeability of polymersomes. ACS Cent. Sci. 2021, 7, 30–38.
Larranaga, A.; Lomora, M.; Sarasua, J. R.; Palivan, C. G.; Pandit, A. Polymer capsules as micro-/nanoreactors for therapeutic applications: current strategies to control membrane permeability. Prog. Mater. Sci. 2017, 90, 325–357.
Leong, J.; Teo, J. Y.; Aakalu, V. K.; Yang, Y. Y.; Kong, H. Engineering polymersomes for diagnostics and therapy. Adv. Healthc. Mater. 2018, 7, 1701276.
Yao, C. Z.; Wang, X. R.; Hu, J. M.; Liu, S. Y. Cooperative modulation of bilayer permeability and microstructures of polymersomes. Acta Polymerica Sinica (in Chinese) 2019, 50, 553–566.
Chang, H. Y.; Lin, Y. L.; Sheng, Y. J.; Tsao, H. K. Structural characteristics and fusion pathways of onion-like multilayered polymersome formed by amphiphilic comb-like graft copolymers. Macromolecules 2013, 46, 5644–5656.
Yan, Q.; Wang, J. B.; Yin, Y. W.; Yuan, J. Y. Breathing polymersomes: CO2-tuning membrane permeability for size-selective release, separation, and reaction. Angew. Chem. Int. Ed. 2013, 52, 5070–5073.
Thambi, T.; Deepagan, V. G.; Ko, H.; Suh, Y. D.; Yi, G. R.; Lee, J. Y.; Lee, D. S.; Park, J. H. Biostable and bioreducible polymersomes for intracellular delivery of doxorubicin. Polym. Chem. 2014, 5, 4627–4634.
Chen, S.; Qin, J. L.; Du, J. Z. Two principles for polymersomes with ultrahigh biomacromolecular loading efficiencies: acid-induced adsorption and affinity-enhanced attraction. Macromolecules 2020, 53, 3978–3993.
Varlas, S.; Foster, J. C.; Georgiou, P. G.; Keogh, R.; Husband, J. T.; Williams, D. S.; O’Reilly, R. K. Tuning the membrane permeability of polymersome nanoreactors developed by aqueous emulsion polymerization-induced self-assembly. Nanoscale 2019, 11, 12643–12654.
Gaitzsch, J.; Hirschi, S.; Freimann, S.; Fotiadis, D.; Meier, W. Directed insertion of light-activated proteorhodopsin into asymmetric polymersomes from an ABC block copolymer. Nano Lett. 2019, 19, 2503–2508.
Kumar, M.; Grzelakowski, M.; Zilles, J.; Clark, M.; Meier, W. Highly permeable polymeric membranes based on the incorporation of the functional water channel protein Aquaporin Z. Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 20719–20724.
Chen, S.; Lin, S.; Xi, Y. J.; Xiao, Y. F.; Du, J. Z. Polymersomes with inhomogeneous membranes, asymmetrical coronas and fused membranes and coronas. Chin. Sci. Bull. 2020, 65, 2615–2626.
Wang, F. Y. K.; Xiao, J. G.; Chen, S.; Sun, H.; Yang, B.; Jiang, J. H.; Zhou, X.; Du, J. Z. Polymer vesicles: Modular platforms for cancer theranostics. Adv. Mater. 2018, 30, 1705674.
Xiao, Y. F.; Sun, H.; Du, J. Z. Sugar-breathing glycopolymersomes for regulating glucose level. J. Am. Chem. Soc. 2017, 139, 7640–7647.
Wang, F. Y. K.; Gao, J. Y.; Xiao, J. G.; Du, J. Z. Dually gated polymersomes for gene delivery. Nano Lett. 2018, 18, 5562–5568.
Zhu, Y. Q.; Wang, F. Y. K.; Zhang, C.; Du, J. Z. Preparation and mechanism insight of nuclear envelope-like polymer vesicles for facile loading of biomacromolecules and enhanced biocatalytic activity. ACS Nano 2014, 8, 6644–6654.
Yang, Y. Y.; Chen, L. S.; Sun, M.; Wang, C. Y.; Fan, Z.; Du, J. Z. Biodegradable polypeptide-based vesicles with intrinsic blue fluorescence for antibacterial visualization. Chinese J. Polym. Sci. 2021, 39, 1412–1420.
Liang, R.; Chen, Y. C.; Zhang, C. Q.; Yin, J.; Liu, X. L.; Wang, L. K.; Kong, R.; Feng, X.; Yang, J. J. Crystallization behavior of biodegradable poly(ethylene adipate) modulated by a benign nucleating agent: zinc phenylphosphonate. Chinese J. Polym. Sci. 2017, 35, 558–568.
Bicerano, J. Prediction of the properties of polymers from their structures. J. Macromol. Sci., Rev. Macromol. Chem. Phys. 1996, C36, 161–196.
Altevogt, P.; Evers, O. A.; Fraaije, J. G. E. M.; Maurits, N. M.; van Vlimmeren, B. A. C. The MesoDyn project: software for mesoscale chemical engineering. J. Mol. Struct.: THEOCHEM 1999, 463, 139–143.
Jiang, J. H.; Zhu, Y. Q.; Du, J. Z. Challenges and perspective on ring-opening polymerization-induced self-assembly. Acta Chim. Sin. 2020, 78, 719–724.
Gartner, T. E.; Jayaraman, A. Modeling and simulations of polymers: a roadmap. Macromolecules 2019, 52, 755–786.
Zhu, Y. L.; Lu, Z. Y. Dynamics simulations of supramolecular and polymeric self-assemblies. Acta Polymerica Sinica (in Chinese) 2021, 52, 884–897.
Zhao, R.; Zhou, Y. J.; Jia, K. C.; Yang, J.; Perrier, S.; Huang, F. H. Fluorescent supramolecular polymersomes based on pillararene/paraquat molecular recognition for pH-controlled drug release. Chinese J. Polym. Sci. 2020, 38, 1–8.
Acknowledgments
J. D. is financially supported by the National Natural Science Fund for Distinguished Young Scholars (No. 21925505) and Shanghai International Scientific Collaboration Fund (No. 21520710100). E. J. C. is supported by the National Natural Science Foundation of China (No. 2210010521), the China Postdoctoral Science Foundation (No. 2020M671197) and International Postdoctoral Exchange Fellowship Program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Notes
The authors declare no competing financial interest.
Electronic Supplementary Information
Rights and permissions
About this article
Cite this article
Chen, S., Cornel, E.J. & Du, JZ. Controlling Membrane Phase Separation of Polymersomes for Programmed Drug Release. Chin J Polym Sci 40, 1006–1015 (2022). https://doi.org/10.1007/s10118-022-2683-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10118-022-2683-7