Abstract
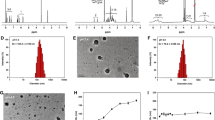
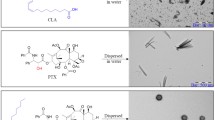
Oleanolic acid (OA) is a pentacyclic triterpenoid compound with extensive biological effects, such as anti-inflammatory and anticancer activities. However, the application of OA in chemotherapy is hampered by its poor solubility and severe adverse effects. To solve the problems, we developed a self-assembled nanoparticle platform based on amphiphilic oleanolic acid polyprodrug, poly[oligo(ethylene glycol) methyl ether methacrylate]-b-poly[oleanolic acid methacrylate] (POEGMA-b-POAMA), encapsulating 10-hydroxycamptothecin (HCPT) to achieve efficient cancer therapy. The polyprodrug was prepared via reversible addition-fragmentation chain transfer polymerization (RAFT), and could self-assemble to prepare POEGMA-b-POAMA/HCPT nanoparticles (NPs). The obtained nanoparticles exhibited appropriate particle size, excellent drug stability, good drug loading capacity, and high drug loading efficiency. In vitro drug release indicated that the drug release was prolonged to 132 h. The POEGMA-b-POAMA/HCPT NPs enhanced cell cytotoxicity in 4T1 cells and MCF-7 cells and could be efficiently uptaken by 4T1 cells. Furthermore, in vivo antitumor efficiency showed that the POEGMA-b-POAMA/HCPT NPs had great antitumor efficiency with considerably low adverse effects in the treatment of the 4T1 mouse breast tumor xenograft tumor. Therefore, POEGMA-b-POAMA/HCPT NPs provide great potential as a platform for drug delivery applications.
Similar content being viewed by others
References
Tao, X. G.; Gou, J. X.; Zhang, Q. Y.; Tan, X. Y.; Ren, T. Y.; Yao, Q.; Tian, B.; Kou, L. F.; Zhang, L.; Tang, X., Synergistic breast tumor cell killing achieved by intracellular co-delivery of doxorubicin and disulfiram via core-shell-corona nanoparticles. Biomater. Sci., 2018, 6, 1869–1881.
Gao, S. T.; Tang, G. S.; Hua, D. W.; Xiong, R. H.; Han, J. Q.; Jiang, S. H.; Zhang, Q. L.; Huang, C. B., Stimuli-responsive bio-based polymeric systems and their applications. J. Mater. Chem. B, 2019, 7, 709–729.
Pei, P.; Sun, C. Y.; Tao, W.; Li, J.; Yang, X. Z.; Wang, J., ROS-sensitive thioketal-linked polyphosphoester-doxorubicin conjugate for precise phototriggered locoregional chemotherapy. Biomaterials, 2019, 188, 74–82.
Gupta, P. K.; Pappuru, S.; Gupta, S.; Patra, B.; Chakraborty, D.; Verma, R. S., Self-assembled dual-drug loaded core-shell nanoparticles based on metal-free fully alternating polyester for cancer theranostics. Mater. Sci. Eng. C-Mater. Biol. Appl., 2019, 101, 448–463.
Kumari, A.; Yadav, S. K.; Yadav, S. C., Biodegradable polymeric nanoparticles based drug delivery systems. Colloid Surf. B-Biointerfaces, 2010, 75, 1–18.
Oh, J. K., Disassembly and tumor-targeting drug delivery of reduction-responsive degradable block copolymer nanoassemblies. Polym. Chem., 2019, 10, 1554–1568.
Dong, S. X.; Sun, Y.; Liu, J.; Li, L.; He, J. L.; Zhang, M. Z.; Ni, P. H., Multifunctional polymeric prodrug with simultaneous conjugating camptothecin and doxorubicin for pH/reduction dual-responsive drug delivery. ACS Appl. Mater. Interfaces, 2019, 11, 8740–8748.
Wang, X. M.; Xu, K. Y.; Yao, H. C.; Chang, L. M.; Wang, Y.; Li, W. J.; Zhao, Y. L.; Qin, J. L., Temperature-regulated aggregation-induced emissive self-healable hydrogels for controlled drug delivery. Polym. Chem., 2018, 9, 5002–5013.
Pound-Lana, G. E. N.; Garcia, G. M.; Trindade, I. C.; Capelari-Oliveira, P.; Pontifice, T. G.; Vilela, J. M. C.; Andrade, M. S.; Nottelet, B.; Postacchini, B. B.; Mosqueira, V. C. F., Phthalocyanine photosensitizer in polyethylene glycol-block-poly(lactide-co-benzyl glycidyl ether) nanocarriers: probing the contribution of aromatic donor-acceptor interactions in polymeric nanospheres. Mater. Sci. Eng. C-Mater. Biol. Appl., 2019, 94, 220–233.
Zabihi, F.; Koeppe, H.; Achazi, K.; Hedtrich, S.; Haag, R., One-pot synthesis of poly(glycerol-co-succinic acid) nanogels for dermal delivery. Biomacromolecules, 2019, 20, 1867–1875.
Liang, K.; Chung, J. E.; Gao, S. J.; Yongvongsoontorn, N.; Kurisawa, M., Highly augmented drug loading and stability of micellar nanocomplexes composed of doxorubicin and poly(ethylene glycol)-green tea catechin conjugate for cancer therapy. Adv. Mater., 2018, 30, 8.
You, C. Q.; Wu, H. S.; Gao, Z. G.; Sun, K.; Chen, F. H.; Tao, W. A.; Sun, B. W., Subcellular co-delivery of two different site-oriented payloads based on multistage targeted polymeric nanoparticles for enhanced cancer therapy. J. Mater. Chem. B, 2018, 6, 6752–6766.
Shao, L. H.; Wan, K. W.; Wang, H.; Cui, Y. K.; Zhao, C. Y.; Lu, J. Q.; Li, X. L.; Chen, L.; Cui, X. Y.; Wang, X.; Deng, X. W.; Shi, X. H.; Wu, Y., A non-conjugated polyethylenimine copolymer-based unorthodox nanoprobe for bioimaging and related mechanism exploration. Biomater. Sci., 2019, 7, 3016–3024.
Yao, J. K.; Kang, S. S.; Zhang, J.; Du, J.; Zhang, Z.; Li, M., Amphiphilic near-infrared conjugated polymer for photothermal and chemo combination therapy. ACS Biomater. Sci. Eng., 2017, 3, 2230–2234.
Matsumura, Y.; Maeda, H., A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res., 1986, 46, 6387–6392.
Zhou, L. Y.; Qiu, T.; Lv, F. T.; Liu, L. B.; Ying, J. M.; Wang, S., Self-assembled nanomedicines for anticancer and antibacterial applications. Adv. Healthc. Mater., 2018, 7, 29.
Pierini, F.; Nakielski, P.; Urbanek, O.; Pawlowska, S.; Lanzi, M.; De Sio, L.; Kowalewski, T. A., Polymer-based nanomaterials for photothermal therapy: from light-responsive to multifunctional nanoplatforms for synergistically combined technologies. Biomacromolecules, 2018, 19, 4147–4167.
Tao, R.; Gao, M.; Liu, F.; Guo, X. L.; Fan, A. P.; Ding, D.; Kong, D. L.; Wang, Z.; Zhao, Y. J., Alleviating the liver toxicity of chemotherapy via pH-responsive hepatoprotective prodrug micelles. ACS Appl. Mater. Interfaces, 2018, 10, 21836–21846.
Ma, X. Q.; Shi, X. X.; Bai, S.; Gao, Y. E.; Hou, M. L.; Han, M. Y.; Xu, Z. G., Acid-activatable doxorubicin prodrug micelles with folate-targeted and ultra-high drug loading features for efficient antitumor drug delivery. J. Mater. Sci., 2018, 53, 892–907.
Dai, L.; Cao, X.; Liu, K. F.; Li, C. X.; Zhang, G. F.; Deng, L. H.; Si, C. L.; He, J.; Lei, J. D., Self-assembled targeted folate-conjugated eight-arm-polyethylene glycol-betulinic acid nanoparticles for co-delivery of anticancer drugs. J. Mater. Chem. B, 2015, 3, 3754–3766.
Palvai, S.; Anandi, L.; Sarkar, S.; Augustus, M.; Roy, S.; Lahiri, M.; Basu, S., Drug-triggered self-assembly of linear polymer into nanoparticles for simultaneous delivery of hydrophobic and hydrophilic drugs in breast cancer cells. ACS Omega, 2017, 2, 8730–8740.
Zhou, Z.; Ma, X.; Jin, E.; Tang, J.; Sui, M.; Shen, Y.; van Kirk, E. A.; Murdoch, W. J.; Radosz, M., Linear-dendritic drug conjugates forming long-circulating nanorods for cancer-drug delivery. Biomaterials, 2013, 34, 5722–5735.
Yar, Y.; Khodadust, R.; Akkoc, Y.; Utkur, M.; Saritas, E. U.; Gozuacik, D.; Acar, H. Y., Development of tailored SPION-PNIPAM nanoparticles by ATRP for dually responsive doxorubicin delivery and mr imaging. J. Mater. Chem. B, 2018, 6, 289–300.
Cheng, G. Q.; Xu, D.; Lu, Z. Y.; Liu, K., Chiral self-assembly of nanoparticles induced by polymers synthesized via reversible addition-fragmentation chain transfer polymerization. ACS Nano, 2019, 13, 1479–1489.
Guegain, E.; Tran, J.; Deguettes, Q.; Nicolas, J., Degradable polymer prodrugs with adjustable activity from drug-initiated radical ring-opening copolymerization. Chem. Sci., 2018, 9, 8291–8306.
Lomkova, E. A.; Chytil, P.; Janouskova, O.; Mueller, T.; Lucas, H.; Filippov, S. K.; Trhlikova, O.; Aleshunin, P. A.; Skorik, Y. A.; Ulbrich, K.; Etrych, T., Biodegradable micellar HPMA-based polymer-drug conjugates with betulinic acid for passive tumor targeting. Biomacromolecules, 2016, 17, 3493–3507.
Hu, X.; Hu, J.; Tian, J.; Ge, Z.; Zhang, G.; Luo, K.; Liu, S., Polyprodrug amphiphiles: hierarchical assemblies for shape-regulated cellular internalization, trafficking, and drug delivery. J. Am. Chem. Soc., 2013, 135, 17617–17629.
Hu, X.; Liu, G.; Li, Y.; Wang, X.; Liu, S., Cell-penetrating hyperbranched polyprodrug amphiphiles for synergistic reductive milieu-triggered drug release and enhanced magnetic resonance signals. J. Am. Chem. Soc., 2015, 137, 362–368.
Hu, X. L.; Zhai, S. D.; Liu, G. H.; Xing, D.; Liang, H. J.; Liu, S. Y., Concurrent drug unplugging and permeabilization of polyprodrug-gated crosslinked vesicles for cancer combination chemotherapy. Adv. Mater., 2018, 30, 7.
Zhu, K. N.; Liu, G. H.; Hu, J. M.; Liu, S. Y., Near-infrared light-activated photochemical internalization of reduction-responsive polyprodrug vesicles for synergistic photodynamic therapy and chemotherapy. Biomacromolecules, 2017, 18, 2571–2582.
Tan, J. J.; Deng, Z. Y.; Liu, G. H.; Hu, J. M.; Liu, S. Y., Anti-inflammatory polymersomes of redox-responsive polyprodrug amphiphiles with inflammation-triggered indomethacin release characteristics. Biomaterials, 2018, 178, 608–619.
Zhang, W. J.; Hu, X. L.; Shen, Q.; Xing, D., Mitochondria-specific drug release and reactive oxygen species burst induced by polyprodrug nanoreactors can enhance chemotherapy. Nat. Commun., 2019, 10, 14.
Pusuluri, A.; Krishnan, V.; Lensch, V.; Sarode, A.; Bunyan, E.; Vogus, D. R.; Menegatti, S.; Soh, H. T.; Mitragotri, S., Treating tumors at low drug doses using an aptamer-peptide synergistic drug conjugate. Angew. Chem. Int. Ed., 2019, 58, 1437–1441.
Duan, X. P.; Xiao, J. S.; Yin, Q.; Zhang, Z. W.; Yu, H. J.; Mao, S. R.; Li, Y. P., Smart pH-sensitive and temporal-controlled polymeric micelles for effective combination therapy of doxorubicin and disulfiram. ACS Nano, 2013, 7, 5858–5869.
He, Y.; Li, X. L.; Ma, J. K.; Ni, G. L.; Yang, G.; Zhou, S. B., Programmable codelivery of doxorubicin and apatinib using an implantable hierarchical-structured fiber device for overcoming cancer multidrug resistance. Small, 2019, 15, 14.
Qin, J. W.; Wei, X. J.; Chen, H. Y.; Lv, F.; Nan, W. B.; Wang, Y. X.; Zhang, Q. Q.; Chen, H. L., mPEG-g-CS-modified PLGA nanoparticle carrier for the codelivery of paclitaxel and epirubicin for breast cancer synergistic therapy. ACS Biomater. Sci. Eng., 2018, 4, 1651–1660.
Lee, W.; Yang, E. J.; Ku, S. K.; Song, K. S.; Bae, J. S., Anti-inflammatory effects of oleanolic acid on LPS-induced inflammation in vitro and in vivo. Inflammation, 2013, 36, 94–102.
Wang, X.; Ye, X. L.; Liu, R.; Chen, H. L.; Bai, H.; Liang, X.; Zhang, X. D.; Wang, Z.; Li, W. L.; Hai, C. X., Antioxidant activities of oleanolic acid in vitro: possible role of Nrf2 and MAP kinases. Chem. Biol. Interact., 2010, 184, 328–337.
Yu, Z. J.; Sun, W. Z.; Peng, W. B.; Yu, R. L.; Li, G. Q.; Jiang, T., Pharmacokinetics in vitro and in vivo of two novel prodrugs of oleanolic acid in rats and its hepatoprotective effects against liver injury induced by CCl4. Mol. Pharm., 2016, 13, 1699–1710.
Niu, S. W.; Williams, G. R.; Wu, J. R.; Wu, J. Z.; Zhang, X. J.; Zheng, H.; Li, S. D.; Zhu, L. M., A novel chitosan-based nanomedicine for multi-drug resistant breast cancer therapy. Chem. Eng. J., 2019, 369, 134–149.
Fontana, G.; Bruno, M.; Notarbartolo, M.; Labbozzetta, M.; Poma, P.; Spinella, A.; Rosselli, S., Cytotoxicity of oleanolic and ursolic acid derivatives toward hepatocellular carcinoma and evaluation of NF-κB involvement. Bioorg. Chem., 2019, 90, 103054.
Pollier, J.; Goossens, A., Oleanolic acid. Phytochemistry, 2012, 77, 10–15.
Jeong, D. W.; Kim, Y. H.; Kim, H. H.; Ji, H. Y.; Yoo, S. D.; Choi, W. R.; Lee, S. M.; Han, C. K.; Lee, H. S., Dose-linear pharmacokinetics of oleanolic acid after intravenous and oral administration in rats. Biopharm. Drug Disposition, 2007, 28, 51–57.
Jiang, Q. K.; Yang, X. X.; Du, P.; Zhang, H. F.; Zhang, T. H., Dual strategies to improve oral bioavailability of oleanolic acid: enhancing water-solubility, permeability and inhibiting cytochrome P450 isozymes. Eur. J. Pharm. Biopharm., 2016, 99, 65–72.
Ghosh, S.; Kar, N.; Bera, T., Oleanolic acid loaded poly lactic coglycolic acid-vitamin E TPGS nanoparticles for the treatment of leishmania donovani infected visceral leishmaniasis. Int. J. Biol. Macromol., 2016, 93, 961–970.
Zhang, L.; Chen, Y.; Shi, R.; Rang, T. G.; Pang, G. S.; Wang, B. R.; Zhao, Y.; Zeng, X.; Zou, C. X.; Wu, P.; Li, J. Y., Synthesis of hollow nanocages MOF-5 as drug delivery vehicle to solve the load-bearing problem of insoluble antitumor drug oleanolic acid (OA). Inorg. Chem. Commun., 2018, 96, 20–23.
Hornig, S.; Heinze, T.; Becer, C. R.; Schubert, U. S., Synthetic polymeric nanoparticles by nanoprecipitation. J. Mater. Chem., 2009, 19, 3838–3840.
Modi, S.; Anderson, B. D., Determination of drug release kinetics from nanoparticles: overcoming pitfalls of the dynamic dialysis method. Mol. Pharm., 2013, 10, 3076–3089.
Guo, Y. F.; Hao, C. Y.; Wang, X. K.; Zhao, Y. N.; Han, M. H.; Wang, M. C.; Wang, X. T., Well-defined podophyllotoxin polyprodrug brushes: preparation via raft polymerization and evaluation as drug carriers. Polym. Chem., 2017, 8, 901–909.
Xu, M. Z.; Zhang, C. Y.; Wu, J. G.; Zhou, H. G.; Bai, R.; Shen, Z. Y.; Deng, F. L.; Liu, Y.; Liu, J., PEG-detachable polymeric micelles self-assembled from amphiphilic copolymers for tumor-acidity-triggered drug delivery and controlled release. ACS Appl. Mater. Interfaces, 2019, 11, 5701–5713.
Du, J. Z.; Du, X. J.; Mao, C. Q.; Wang, J., Tailor-made dual pH-sensitive polymer-doxorubicin nanoparticles for efficient anticancer drug delivery. J. Am. Chem. Soc., 2011, 133, 17560–17563.
Ke, W. D.; Yin, W.; Zha, Z. S.; Mukerabigwi, J. F.; Chen, W. J.; Wang, Y. H.; He, C. X.; Ge, Z. S., A robust strategy for preparation of sequential stimuli-responsive block copolymer prodrugs via thiolactone chemistry to overcome multiple anticancer drug delivery barriers. Biomaterials, 2018, 154, 261–274.
Lin, J. T.; Ye, Q. B.; Yang, Q. J.; Wang, G. H., Hierarchical bioresponsive nanocarriers for codelivery of curcumin and doxorubicin. Colloids Surfaces B, 2019, 180, 93–101.
Yang, H.; Li, J.; Patel, S. K.; Palmer, K. E.; Devlin, B.; Rohan, L. C., Design of poly(lactic-co-glycolic acid) (PLGA) nanoparticles for vaginal co-delivery of griffithsin and dapivirine and their synergistic effect for HIV prophylaxis. Pharmaceutics, 2019, 11, 184.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 21576029) and National Key R&D Program of China (No. 2017YFD0601205).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, YS., Li, GL., Zhu, SB. et al. A Self-assembled Nanoparticle Platform Based on Amphiphilic Oleanolic Acid Polyprodrug for Cancer Therapy. Chin J Polym Sci 38, 819–829 (2020). https://doi.org/10.1007/s10118-020-2401-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10118-020-2401-2