Abstract
Yalimapo beach, near the Maroni River estuary in French Guiana, is an important turtle nesting site. The interaction of massive mud banks migrating alongshore from the distant Amazon River with discharge from the Maroni River generates strong beach morpho-sedimentary changes. The eventual degradation of the marine turtle nesting habitats resulting from these changes represents a threat to the offspring, and consequently, to the turtle population. Field operator counts of green and leatherback turtle nests were combined with high-resolution topographic measurements of the beach over four field surveys in 2012 and 2014 to map the topographic modifications susceptible to affect nesting on Yalimapo beach. We assumed that the survival of nests was at stake when the depth of sand between the egg chamber and the topographic surface (i.e. the top) of the beach was < 50 cm, and that beach surface lowering > 10 cm represented unfavourable conditions for nest safety with unequal nest survival across the beach. Erosion of the beach surface exceeding a depth of 50 cm therefore results in nest destruction. Digital elevation models were produced to quantify the topographic modification of nesting on Yalimapo beach and highlight the endangered nesting areas. As the modification of the beach is not linear, some sectors are more eroded than others, resulting in unequal nest survival across the beach. Overall, up to 40% of the nests were presumed destroyed over the 2 years of survey, but true losses would depend on the species and the preferential locations of their nesting habitats. The relatively unfavourable conditions that prevailed during the 2 years of the survey are consistent with persistent erosion of Yalimpao beach since 2011. This ongoing erosion could explain in part the drastic decline of the leatherback turtle population in western French Guiana over the period 2001–2018. The substrate quality and dynamics of the nesting beach in relation to the preferred nesting habitat of each species are therefore critical issues that should be considered in the conservation strategies of marine turtles. The beach nesting conditions of marine turtles in French Guiana, as elsewhere, could be further aggravated in the future by climate change effects, including sea-level rise.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Climate change will induce profound changes in biodiversity and ecosystem functioning (Bellard et al. 2012). The most recent scenarios projected by the Intergovernmental Panel on Climate Change (IPCC) show that mean temperature may increase by 4 °C by the end of the twenty-first century (Lee et al. 2021). It is important, therefore, to evaluate the extent to which animals that are particularly sensitive to climate conditions can cope with these changes. The situation could be particularly critical for already threatened species, since the impact of climate change might be the final trigger for their extinction. Climate change is a threat to marine turtles (Patrício et al. 2021) which are classified as Vulnerable or Endangered on the IUCN Red List of Threatened Species (IUCN 2021). Wallace et al. (2011) summarized the various threats faced by marine turtles: fishery bycatch, direct use of turtles or eggs by humans, coastal development resulting in the loss or degradation of nesting habitats, pollution and pathogens, and climate change. The thorough inventory carried out by Wallace et al. (2012) showed that climate change had the second highest threat score, but it was omitted from final calculations and categorizations due to serious data gaps.
Because marine turtles do not provide parental care, their breeding success depends on the characteristics of the sandy beaches used as nesting sites and to which they have a high level of fidelity (Carr and Carr 1972; Weishampel et al. 2003). Turtles dig their nest on the portion of the beach located between the upper intertidal area and the shrub vegetation (Péron et al. 2013). The nest depth depends on the length of the hind flippers of the species: around 50 cm for olive ridleys, 70 cm for green turtles, and 80 cm for leatherbacks (Santidrian Tomillo et al. 2017). The incubation period varies between 45 and 90 days depending on the species and the incubation temperatures (Miller 1997). Marine turtles have temperature-dependent sex determination, and the sex of the embryos thus depends on the temperature experienced during incubation (Mrosovsky and Yntema 1980; Mrosovsky 1994; Hulin et al. 2009). This mechanism of sex determination could render turtles particularly exposed to the impacts of climate change (Maurer et al. 2021). Furthermore, hatching success is also temperature-dependent and temperatures that are too low or too high impair development (Howard et al. 2014).
Coastal systems are highly dynamic environments, and sandy beaches that are the nesting grounds for marine turtles are among the landforms most subject to change (Anthony 2019). Beach morphology can change rapidly in response to storms, which can generate both massive beachface lowering and retreat in hours to days, whereas recovery under lower energy waves may take weeks to months (Anthony 2019). These morphological changes can significantly impact turtle nesting. Changes in nesting site elevation over the incubation chamber induce temperature variations that are known to influence the sex ratio at hatching (Hulin et al. 2009; Maurer et al. 2021). Generally, beach erosion is associated with a decrease in hatching success (Lamont and Carthy 2007; Lamont and Houser 2014; Varela et al. 2019). Beach erosion and inundation further degrade nests (Daniels et al. 1993; Fish et al. 2005; Willis-Norton et al. 2015). Egg exposure and drowning on beaches can be important sources of nest failure (Witherington et al. 2011). During the nesting incubation period (~ 60 days) (Miller 1997), beach dynamics may involve large movements of sand in the nesting areas. Even where beach erosion is not sufficient to directly result in the loss of nests, changing beach dynamics may indirectly contribute to their loss. Erosion renders the nests more accessible to predators such as birds, crabs, raccoons, and dogs (Engeman et al. 2006). Like other animal species relying on the coastal habitat for nesting, marine turtles also face the impact of sea-level rise on beaches. Beach erosion is expected to intensify, leading to a predicted decline in nesting grounds for marine turtles (Fish et al. 2005; Varela et al. 2019). As sea level is also expected to rise considerably during this century, the nesting sites will also be exposed to the deleterious effects of increase in moisture and flooding (Baker et al. 2006; Fuentes and Abbs 2010; Fuentes-Farias et al. 2010).
Sandy beaches in French Guiana, South America (Fig. 1) are major nesting sites for three of the seven species of marine turtles: leatherback (Dermochelys coriacea), green turtle (Chelonia mydas), and olive ridley (Lepidochelys olivacea) (Baudouin et al. 2015; Chambault et al. 2016a, 2016b, 2017, 2015). The IUCN Red List categorizes green turtles as Endangered (Seminoff 2004; The Second World Ocean Assessment 2021), and leatherbacks and olive ridleys as Vulnerable (Abreu-Grobois and Plotkin 2008). In French Guiana, leatherback turtles nest in open sandy beach foreshore areas (Caut et al. 2006a, 2006b; Kamel and Mrosovsky 2004; Péron et al. 2013; Whitmore and Dutton 1985) and green turtles in beach backshore vegetation (Péron et al. 2013). The sandy beaches of French Guiana are, however, highly dynamic (Anthony et al. 2010; Jolivet et al. 2019a), and nest fidelity jeopardizes the persistence of populations of marine turtles (Kelle et al. 2007; UICN France and Museum d’Histoire Naturelle de France 2017). This is notably the case of Yalimapo beach, in western French Guiana (Fig. 1). These beaches are associated with large discrete nearshore mud banks, separated by inter-bank areas, that migrate westward under the influence of wave-generated processes from the mouths of the Amazon River in Brazil to those of the Orinoco in Venezuela (Anthony et al. 2010; Gardel and Gratiot 2005). By modulating alongshore wave energy dissipation and refraction, mud banks and alongshore-alternating inter-bank phases can lead to important beach morphological modifications that are further amplified by the interaction of mud and sand (Anthony et al. 2019; Jolivet et al. 2019a). These morphological modifications can significantly impact nesting success. Many nesting females that return to lay their eggs commonly face eroded or inaccessible beaches, beaches that no longer meet the criteria for successful incubation, or newly formed ‘chenier’ beaches (i.e. beach deposits pushed inland by waves and wave overwash within the muddy coastal plain) that are subject to high mobility and intermixture with mud (Anthony et al. 2013; Brunier et al. 2022). These morphological variations in turtle nesting areas in western French Guiana can be expected to lead to the deterioration of the incubation conditions of the eggs.
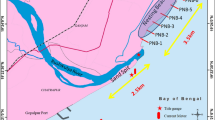
The Guianas coast between the deltas of the Amazon and the Orinoco Rivers in South America (B) and inset showing 3-h averages of significant wave height (Hs) derived from a record of data extracted from the model WaveWatch III (NOAA) offshore of the Maroni (A); Sentinel 2 satellite image showing the location of Yalimapo beach in French Guiana, the neighbouring Maroni River mouth and the current partially shore-welded mud bank (C)
On Yalimapo beach, nest density is unevenly distributed, and some areas show an aggregation of nests and others low nest density (Girondot et al. 2006; Péron et al. 2013). Yalimapo beach has also been shown to be dynamic with marked changes in erosion and accretion over the last five decades (Jolivet et al. 2019a). This beach provides, thus, an opportunity for assessing the impacts of beach morphological characteristics and change on nest safety and vulnerability and for establishing a prognosis of turtle population dynamics in a conservation context.
Materials and methods
Study area
The study was conducted on the 3-km-long Yalimapo beach abutting the east bank of the Maroni River estuary (Fig. 1) within the protected site of the Amana Nature Reserve (Fig. 1). This area is influenced by a tropical-equatorial seasonal climate influenced by the north–south migration of the Intertropical Convergence Zone (ITZC). A well-marked rainy season occurs from the end of December to July and a dry season from August to December. This contrast is characterized by marked variations in seasonal river discharge, with a threefold to fourfold increase in the rainy season. The rainy season is generally interrupted by dry conditions during the month of March. The beach experiences semi-diurnal tides with a spring range of 4.1 m and a neap range of 1 m and is exposed to relatively dissipated ocean waves (mean significant wave height < 40 cm) as a result of the presence of a large estuarine sand bank extending offshore from the mouth of the Maroni (Jolivet et al. 2019a; Gardel et al. 2021). The wave regime is seasonal with relatively energetic deep-water waves prevailing from December to March and lower waves from April to November.
Field data were obtained in the course of surveys carried out over two consecutive periods in 2012 (named 2012A and 2012B) and 2014 (named 2014A and 2014B) involving in situ topographic measurements as well as nest counts (2012A: 12 March–18 May; 2012B: 18 May–11 July; 2014A: 10 April–12 May; 2014B: 12 May–26 July). Observations on the morphology of the beach were also carried out during these surveys.
Spatial distribution of marine turtle nests and site characteristics
A team of four to six operators conducted capture-mark-recapture (CMR) monitoring of leatherback and green turtle nesting populations at night, 4 h before and after high tide. We identified each individual turtle (marked with a passive integrated transponder) coming ashore to nest, and carried out an inventory of activities. The nest position was geolocated with a GPS Etrex 10. The activities noted were as follows: sand-sweeping, digging, egg-laying, and egg-covering. At the same time, the characteristics of the nest habitat were also noted. These characteristics were the presence of bare sand, the boundary between sand and vegetation, the presence of a separation of the lower from the higher foreshore, and of the foreshore from the backshore, and the presence of a retreating beachface foot indicative of erosion.
Geomorphological changes
High-resolution topographic measurements of the beach were carried out using a Leica 4700 Total Station with an instrumental accuracy of ± 3 mm for distance and height and ± 0.0015° for direction. A total of eight Digital elevation models (DEMs) were generated from the raw topographic data at the intervals corresponding to the 2012A, 2012B, 2014A, and 2014B survey intervals mentioned above, i.e. respectively 67, 54, 32, and 76 days. Digital elevation models are mapped representations of topography in 3D mode (showing spatio-temporal variations in elevation). They are important in the visualization of landscape changes over time, and therefore represent an instructive way of depicting how beach changes in space and time affect turtle nests. The dates of the topographic surveys were chosen to coincide with spring tides in order to cover geomorphic changes on as large an area of the beach as possible.
In order to include a time frame, differentials of DEMs (DoDs) representing elevation variations over the beach between two surveys were used to determine levels of erosion and accretion. The DEMs and their differentials were determined for a 1500-m section of the beach (Fig. 2). The DEMs also enabled determination of changes in the width of the beach through mapping of the contact between the bare sandy intertidal foreshore and the vegetated supratidal backshore. The dates of the topographic surveys were chosen to coincide with spring tides, thus enabling monitoring of as large an area of the beach as possible.
Topographic and nest location data were mapped using Arcgis 9.2 software (ESRI Inc.). The data were projected in the WGS 84 UTM 22 North system. Based on the postulate that beach topographic conditions and their eventual changes significantly influence nesting success or vulnerability by affecting the depth of sand between the egg chamber and the beach surface, but also by directly leading to nest erosion we used the Dods over the study period to map the impact of topographic changes, portrayed as beach nest exposure maps. Changes in beach elevation over the incubation chamber denote either erosion (removal of sand) or accretion (accumulation of sand). These changes affect the temperature of the nests and, thus, potential hatching success, but they can also lead to direct removal of nests through beach erosion or indirectly affect nesting success by reducing the beach space available for nesting, a condition that can lead to turtles coming ashore destroying pre-existing nests. We used the following thresholds to categorize elevation change: three of which (> 50 cm, between 40 and 50 cm, and between 10 and 40 cm) correspond to ‘unfavourable’ conditions of elevation lowering (erosion) with potential negative impacts on nesting success (Spanier 2010); changes between –10 and + 10 cm are considered as neutral as they reflect stability of the nesting beach, and we consider, provisionally, as inderterminate regarding the incubation of marine turtles, situations where accretion > 10 cm. The impact of high beach accretion levels has not been identified in this study as a result of the relatively low temporal frequency of the geomorphological surveys. This aspect is revisited in the discussion. The safest nests are defined as those located in the neutral region.
Phenology of marine turtle nesting
Abundance of turtle nests
The abundance of turtle nests was calculated using a seasonal abundance model (Girondot 2010, 2017; Girondot and Rizzo 2015). The abundance model describes the nesting season using shape and scale parameters. Shape parameters are B (for Beginning) for the ordinal date of the start of the nesting season, E (for End) for the ordinal date of the end of the nesting season, and P (for Peak) for the ordinal date when the peak of the nesting season is observed (Girondot 2010). More convenient formulations are LB = P-B and LE = E-P where LB and LE are > 0, and LB and LE are abbreviations for Length of Beginning and Length of End, respectively. The scale parameters are Max and Min, which are respectively the number of nests at the date of the peak and the number of nests before date B or after date E (i.e. out of the nesting season). A more convenient parametrization uses \(P\mathrm{Min}\) with \(\mathrm{Min}=P\mathrm{Min}\times \mathrm{Max}\), which has the advantage of using only one parameter \(P\mathrm{Min}\) if the model is applied for several time series of counts, with the hypothesis that the daily number of nests out of the nesting season is proportional to the average number of nests at the peak of the season (\(\mathrm{Max}\)).
The estimation of the value of the parameters that represent the best observations was done using the maximum likelihood with a negative binomial distribution with an aggregation parameter Theta (Girondot 2017). Theta represents the day-by-day dispersion of the observations below and above the model. The distribution of the parameters is obtained using the Metropolis–Hastings algorithm, which is a Markov chain Monte Carlo (MCMC) method for obtaining a sequence of random samples from a probability distribution (Hastings 1970; Metropolis et al. 1953). This method is now widely used, as it offers a high-performance tool to fit a model. Wide uniform priors are chosen to ensure that they do not constrain the posteriors. The adaptive proposal distribution (Rosenthal 2011) as implemented in R package HelpersMG (Girondot 2021) ensures that the acceptance rate is close to 0.234, which is the optimal acceptance rate (Roberts and Rosenthal 2001). The starting values for the MCMC are the maximum likelihood estimators. The total number of iterations required was defined after an initial run of 10,000 iterations (Raftery and Lewis 1992).
Consequences of erosion on hatchling production and sex determination
Using the distribution of nests during the 2012 and 2014 nesting seasons, the median date of the nesting season was estimated for both species. The proportion of nests in stable zones (change in elevation between –10 and + 10 cm) was estimated by superimposing the distribution of nests during the nesting season on the digital elevation models. The median date of a phenology is the date for which 50% of nests are located prior to this date and 50% after. The median dates for 2012 and 2014 for the distribution of nests were estimated together with the median date for only the nests located in the stable zone. The change in these median dates was reported as a function of the temperature at the depth of the nests. Temperatures at various nest depths (28–100 cm; variable stl3) were extracted from the European Centre for Medium-Range Weather Forecasts ERA-5 database at the nearest location (longitude –54°, latitude 5.5°; WGS 84). ERA5 is the fifth generation ECMWF atmospheric reanalysis of the global climate covering the period from January 1950 to present (COPERNICUS 2017). ERA5 provides hourly estimates of a large number of atmospheric, land, and oceanic climate variables using 4D-Var data assimilation and model forecasts. The data cover the Earth on a 0.36-degree grid with a temporal resolution of 1 h and resolve the atmosphere using 137 levels from the surface up to a height of 80 km. ERA5 includes information about uncertainties for all variables at reduced spatial and temporal resolutions. Ground temperature in the ERA5 database is a good approximation of sand temperature at the level of nests (Patrício et al. 2021).
Results
Beach geomorphological changes
Yalimapo beach exhibited throughout the study period a classical beach profile that ranged from reflective to dissipative in terms of the beach morphodynamic signature (Jolivet et al. 2019a). The beach comprised a sandy intertidal foreshore forming the beachface, separated by either a more or less well-expressed accretionary berm or an erosional bluff from a vegetated backshore dominated by creeping grasses, notably Ipomoea pes-caprae. Beach width at low spring tide ranged from 60 to 75 m, and the sandy beachface invariably merged at about the spring low tide mark with a muddy shoreface expressing the abundance of mud and the pervasive influence of mud banks on this coast. A notable exception to this was in a small sector of the western part of the beach (Eastings 173,400 to 17,366, Fig. 2a) where there is a permanent shore-attached sand shoal exhibiting large tide-driven hydraulic dunes associated with the Maroni estuary (Jolivet et al. 2019a). Since no measurements were carried out in 2013, a DEM comparison was made over the 2-year period between the comparable months of March 2012 and April 2014 (Fig. 2a). Although this 25-month period should incorporate seasonal beach morphological variation (Jolivet et al. 2019a) involving the classical beach ‘cut’ (higher waves from December to March and beach erosion) and ‘fill’ (lower waves from April to November and beach recovery), change was marked alongshore. Erosion, expressed by a decrease in beach elevation, dominated largely, and was especially severe in much of the eastern sector as well as the western extremity close to the Maroni estuary. These two erosional sectors were separated by a short 150-m central sector, associated with the permanent shore-attached shoal, characterized by accretion. Elevation change attained as much as − 3.4 m in the eroded sectors, whereas this short segment of the beach gained up to 2.2 m in elevation (Fig. 2a). These morphological modifications were associated with variations in the beach profile. The accretionary segment showed a convex beachface with a poorly expressed berm, corresponding, thus, to dissipative conditions. In contrast, a concave profile prevailed along much of the eroded sectors of the beach, resulting in a more reflective beachface often characterized by steep slopes (> 20°), and a vertical bluff where the berm was being removed by erosion. The measurements also showed that morphological changes sometimes occurred over a very short period of time, as shown by a DEM differential between March and May, 2012 (Fig. 2b) depicting, this time, changes in beach width expressed as cross-shore mobility of the vegetation contact separating the upper foreshore and the backshore. This period corresponds to the waning phase of the high wave-energy season during which Yalimapo beach undergoes erosion. In some sectors, the vegetation line retreated by up to 5 m, notably in short segments at the eastern extremity of the beach (Fig. 2b).
Nesting and hatchling production
We counted 554 and 410 nests of, respectively, green turtles and leatherbacks in the course of the first survey of 2012 (2012A: 12 March–18 May), respectively 116 and 556 nests in the second (2012B: 18 May–11 July), and respectively 387 and 251 nests in the first survey of 2014 (2014A: 10 April–12 May), and 383 and 784 nests in the second (2014B: 12 May–26 July 2014). The daily nest counts for both green and leatherback turtles as well as the model of the nesting season are shown in Supplementary Table 1 and Supplementary Fig. 1. Green turtle nests were much more abundant in 2012A compared to 2012B (554 vs. 116) but the counts were comparable for the two 2014 periods (387 vs. 383). For leatherback turtles, counts were more important for the second nesting period in both years (2012A and B: 410 vs. 556; 2014A and B: 251 vs. 784). The fitted models of the nesting season for both species are summarized in the grey boxes in Fig. 3, and in Supplementary Fig. 2. The temperature at depths of 28–100 cm is also shown in Fig. 3. A temperature increase occurred in relation to the transitions from the rainy to the dry season (centred around March) the dry to the rainy season (centred around November) for both periods. The proportion of safe nests (located in the stable, neutral zones) in periods A and B is only slightly different from the total distribution of nests during the nesting season (Fig. 3).
Hourly temperatures at 28–100 cm in 2012 (A) and 2014 (B) (longitude − 55°, latitude 5.5°; WGS 84) along with the nesting season and distribution of safe nests (–10 to + 10 cm; see Supplementary Tables 1 and 2) for leatherback and green turtles. The light and dark grey boxes are respectively the [2.5%; 97.5%] and [25%; 75%] quantiles; dashed lines are the medians of the distributions
Maps of nest susceptibility to loss
Four maps were prepared from the DoDs in order to assess the degree to which nests on Yalimapo beach were potentially exposed or not to loss resulting from erosion based on the three areal categories of threat (red), danger (orange), and vulnerability (yellow), collectively representing ‘unfavourable’ conditions. Neutral conditions were associated with relative beach topographic stability (white), whereas indeterminate conditions (green) correspond to net beach surface accretion exceeding 10 cm (Fig. 4). Map 2012A, representative of the period from March to May 2012, shows significant comparable proportions of nests exposed to the uppermost unfavourable levels of ‘threat’ and ‘danger’ (green: 15.4%; leatherback: 16.3%). Map 2012B, covering May to July 2012, shows a less dynamic beach system with much lower ‘threat’ and ‘danger’ exposures for the nests (green: 3.5%; leatherback: 2.9%), corresponding to conditions of seasonal beach ‘fill’ (recovery). Map 2014A shows a much more dynamic beach with accretion largely superseded by beach erosion, consistent with the high-energy season, and generating notably sites in danger, especially towards the western extremity, and under threat at the eastern extremity of the beach. The last map (2014B), May to July 2014, depicts a relatively similar situation to that of the 2012B map, with accretion largely dominant across the entire beach (low-energy beach recovery season) except in the central sector where elevation diminished.
Differential of DEMs of Yalimapo beach from 2012 to 2014 showing changes in beach elevation expressed as susceptibility maps of turtle nests to unfavourable (erosion), neutral (stability) or indeterminate (accretion > 10 cm) conditions. Safe nests are associated with neutral beach elevation changes. Left panels represent the cumulative proportions
To summarize, during the 2012 and 2014 nesting seasons, we monitored the following mean and standard deviation values associated with the erosion, stability, and accretion levels of the beach: 28.4% ± 3.9% of green turtle nests were found in areas subject to erosion (from 10 to > 50 cm), 46% ± 14.2% in a stable area (–10 to + 10 cm), and 25.7% ± 6.9% in an accreting area. The estimated proportion of green turtle nests considered lost (i.e. erosion > 50 cm) was 6.6% (n = 44) in 2012 and 4.8% (n = 54) in 2014. However, 57.6% (n = 444) of the nests were located in stable areas of the beach in 2014. Beach morphological change was more pronounced in 2012 and only 31.3% of nests (n = 210) were located in stable areas. Overall, ‘unfavourable’ conditions were more common for leatherbacks than for green turtles, tantamount to more important potential losses during the two survey years.
An average of 36.0% ± 11.7% of nests were located on portions of the beach characterized by accretion. This does not mean that the beach is generally in accretion, but rather that many leatherback nests are located in the backshore area beyond the beach scarp, which can be unstable and collapse during high spring tides or when a turtle digs a nest there. Most leatherback nests in 2012 and 2014 were located in stable or accreting areas, which accounted for 31.8% (n = 307) and 31.3% (n = 302) of nests, respectively. Accreting areas showed more leatherback nests during the 2014 nesting season, as 46.6% (n = 482) of these nests were located in this zone.
Discussion
In this study, we have focused essentially on nest susceptibility to erosion as this process can not only be directly detrimental to eggs, removing them as beach sediment is evacuated, but has also been dominant over the study period on Yalimapo beach (Fig. 2). Although accretion is favourable to a healthy beach sediment budget, it may, depending on its intensity, lead to diminished turtle hatching and emergence success as the hatchlings are obliged to dig up more sand to emerge, or to cover a longer distance that may put them in greater jeopardy from predators, before they attain the sea. We have mapped beach zones where accretion exceeded 10 cm as sectors of ‘indeterminate effects’. These sectors cover a much lower area of Yalimapo beach than erosion sectors over the study period (Fig. 2a). The data show that the proportion of nests susceptible to be impacted by beach erosion differed in 2012 and 2014 for both green and leatherback turtles, depending on the eastward to westward variation in beach topographic change (Fig. 4) and along the beach profile (Fig. 2b). Because of the prevalence of erosion over the period 2012–2014, much of the beach was exposed to low potential hatching success. We assume, however, that significant accretion attaining up to 2 m, for instance, such as in parts of the shore-attached sand shoal in Fig. 2a, could also pose a problem to hatching success for the reasons evoked above. We discuss, first, a number of limitations imposed on our findings by the methods we have employed and how these can be improved, and then the eventual adaptation of turtle nesting to beach erosion as well as the causes of now persistent erosion of Yalimapo beach and the alarming perspectives this spells for marine turtle populations in French Guiana.
Methodological limitations and potential improvements
Whereas the topographic surveys using a total station had a very high distance and elevation resolution (± 3 mm), the surveys on nest distribution were carried out with a GPS Etrex 10 (with GPS and GLONASS satellites for rapid positioning), a system subject to relatively marked fluctuations in position accuracy (> 3–5 m). Combined nest and beach topographic survey resolution and survey frequency are now considerably enhanced using Differential Global Positioning Systems (DGPS), especially when coupled with photogrammetric techniques based on UAVs or drones (e.g. Brunier et al. 2016a, 2016b; Varela et al. 2019). This is especially pertinent to the French Guiana coast where changes can be rapid and where very high-resolution drone-based geomorphic monitoring is particularly suitable (Brunier et al. 2020). With regard to these geomorphic surveys and the highly dynamic nature of beach changes in French Guiana, beach survey frequency should optimally be carried out as frequently as possible (even on a daily basis during the nesting season) in order to capture nest locations and enhance the monitoring resolution of the topographic changes affecting nests. There is a possibility that the proportion of nests destroyed was underestimated as a result of the low geomorphic survey frequency employed in this study. Another point concerns the simulation of temperatures at different nest depths (28–100 cm; variable stl3) via the ERA database which involves hourly estimates of a large number of atmospheric, terrestrial, and oceanic climate variables on a 0.36-degree grid, with a temporal resolution of one hour. Although ground temperature in the ERA5 database is a good approximation of the temperature of the sand at the level of the nests (Patrício et al. 2021), uncertainties will necesarilly subsist regarding temperature precision using model data compared to actual temperatures in the nests, which, it should be recalled, are subject to the influence of various environmental factors. Finally, determining the viability of nests also requires data on various biotic (e.g. temperature, humidity, salinity) and abiotic (e.g. slope of the beach, vegetation, accretion levels > 10 cm, grain size, frequency and duration of flooding caused by tide and waves) factors, some of which have not been monitored in the course of our study. Thus, in order to estimate the viability of nests, we recommend, in parallel with higher-frequency and higher-resolution erosion/accretion surveys and measurement of the biotic and abiotic factors mentioned above: (1) the installation of temperature and humidity data loggers in the nests (e.g. Tinytag Plus 2), in order to obtain high-resolution in situ data on temperature and humidity, and (2) egg-counting during the laying process (on about 100 nests distributed over the entire laying site) in order to obtain data on hatching and emergence success, which as mentioned above, is impaired by erosion but also potentially by massive accretion. Whereas studies on turtle vulnerability to beach morphological change have essentially concerned beach erosion, there is a need for more studies on the impacts of rapid and significant acreetion too, as this can affect hatching success. Having said this, however, we believe that the data generated in this study are robust enough to provide a useful assessment of the impact of beach dynamics on turtle nesting and vulnerability, and that the methodological guidelines suggested above will foster more high-resolution studies of the impact of beach dynamics on turtle nesting.
Potential turtle nest losses and nesting adaptation to beach erosion
In terms of a comparison of the 2 years, 2012 was a more erosive year on Yalimapo beach, notwithstanding lower deep-water waves (Fig. 1). Our data show that the more severe erosion, especially of the eastern half of the beach between March and May 2012 (Fig. 4d), which led to both elevation decrease and a retreat of several decimeters of the backshore vegetation contact line with the upper beach, was associated with a decrease in the number of nests at the foot of the beach scarp for both leatherback and green turtles. We estimated that during 2012, up to 36% of both leatherback and green turtle nests may have been lost as a result of the prevalence of erosion, especially in the eastern sector of the beach. These estimated losses are to be scaled against the much larger number of nests (Table 1) in the course of the first nesting period of March to May 2012 (83%), the higher wave-energy season, compared to the period May to July (17%) when the beach showed significant recovery (Fig. 4b). Laying at the foot of the beach scarp, which is, in itself, a typical beach erosional feature, could increase erosion and nest loss. Nest construction within this unstable zone can lead to beach sand collapse or can promote nest overwashing in the course of the next high tide. Figure 2b shows that large parts of this contact retreated significantly between 2012 and 2014. The vegetated supratidal part of the beach remained the most stable, as wave action was limited to the more fluctuating sandy intertidal foreshore between low and high spring tides. A comparison of the 2 years 2012 and 2014 highlighted an increase, from 31.4 to 57.6%, of green turtle nests in stable areas, essentially the backshore of the beach, which is generally above the level of wave action at high tide. These observations suggest that both species of marine turtles adapt their nesting behaviour to the beach dynamics, with green turtles preferably nesting on the vegetated backshore areas, in agreement with findings from previous studies (Bustard and Greenham 1968; Péron et al. 2013). We also draw the logical conclusion that the more stable the beach, as in 2014, albeit with a net sand loss compared to 2012 (Fig. 2a), the more successful the nesting. The prevalence of erosion in the 2012–2014 differential (Fig. 2a) was a signal that antecedent erosion was becoming persistent on Yalimapo beach, a point further confirmed below. This again suggests some adaptation of the nesting behaviour to the prevailing state of the beach (Table 2).
Although Yalimapo beach experiences a subdued wave regime due to offshore energy dissipation, the interaction of mud with waves, and locally with river discharge, generates, as Jolivet et al. (2019a, 2019b) showed recently, complex nearshore processes. These can, in turn, lead to significant beach erosion and accretion phases that have been shown to prevail over sub-decadal to multi-decadal periods. The deep-water wave conditions offshore of Yalimapo over the period 2010–2018 (Fig. 1) suggest that this overall prevalent erosion is not readily linked to variations in wave energy. Erosion can occur even when waves are of relatively lower amplitude (as in 2012 compared to 2014), because the interaction of sand with mud rendered more fluid by water discharge from the nearby Maroni River leads to high beach sediment mobility (Jolivet et al. 2019a). This mobility accounts for the significant changes observed during the study period, whether over the 2012–2014 period of observation (Fig. 2a) or over a period incorporating seasonal changes (Fig. 2b). The implications of this situation are rather alarming for the sustainability of marine turtle nesting on Yalimapo beach, given the generalized erosion, but also accretion (up to 2 m) that could be unfavourable to emergence success in the shore-attached shoal area, the only largely accreting zone on this beach (Fig. 2a). We presume, however, that in this restricted accreting area, nests located on the uppermost part of the foreshore undergo less accretion and could, therefore, be favourable to successful hatching. Over the rest of Yalimapo beach, nests on the beach scarp or even located in the backshore close to the vegetation line were more likely to be eroded. The beach is therefore in a situation in-between the potential to host safe nests, but only in restricted favourable sites, and its susceptibility to erosion on other parts that were, paradoxically, more frequented by turtles simply because they represented much larger stretches of beach.
Onset of chronic beach erosion and declining leatherback turtle populations
French Guiana beaches represent a globally important nesting site for leatherbacks (Fretey and Lescure 1998; Girondot and Fretey 1996). While leatherbacks nesting on French Guiana beaches once represented as much as 40% of the world’s leatherbacks (Girondot et al 2006), there has clearly been a severe decline in recent years, as shown by long-term population monitoring at Yalimapo beach where the proportion now represents only 10%, with 200 annual nests in 2018 vs. 18,000 in 2001 (equivalent to an average decline of 19% year−1, Chevallier et al. 2020). In this context, it seems important to carry out future studies aimed at understanding the mechanisms that affect the approach of sea turtles to a specific nesting site (bathymetry, topography of the beach, substrate, density of individuals, etc.), but also the longer-term dynamics of these nesting sites.
The overarching conservation question on this endangered species is whether this drop in the population is due to a reduction of the area of the nesting habitat and to lesser accessibility of the beach to the nesting marine turtles. Péron (2014) calculated a shoreline mobility envelope of 225 m ± 40 m between 1950 and 2012. Jolivet et al. (2019a), found, however, that net erosion has prevailed on Yalimapo beach between 2011 and 2017, and this tendency is confirmed by ongoing drone surveys of the beach over the last 3 years conducted by CNRS Guyane. This pervasive erosion tendency marks a clear turnaround from accretion that was dominant up to 1987, but that then declined progressively prior to the switch to the current erosional regime since 2011 (Jolivet et al. 2019a). This change has been attributed, according to Jolivet et al. (2019a), to the increasing encroachment of a mud bank (Fig. 1), the leading edge of which is located less than 1 km from the beach, resulting in increased mixing of sand and mud that has been deemed nefarious to turtle nesting (Marco et al. 2017). The effect of the reduction of the nesting habitat, through erosion as a result of the proximity of this mud bank, has led to the increasing concentration of nests in a smaller area of beach (3 km in 2012–2014 vs 1.5 km in 2018, according to our recent field observations), leading inexorably to nest destruction by other congeners (Girondot et al. 2002). The ongoing drone surveys of the beach suggest that the erosion of Yalimapo beach plays out under what has been termed beach ‘rotation’, a process involving lateral transfer of sand to feed accretion elsewhere. In this case, the sand eroded from Yalimapo beach is accumulating east of Yalimapo, feeding the rapid accretion of a chenier at Awala located behind mangroves that are rapidly colonizing a shore-welded part of the mud bank (Fig. 1). This eastward relocation of sand and the ensuing beach geomorphic and sedimentary configuration in a mud-rich setting are not favourable to turtle landings and nesting. Beach rotation in French Guiana is generated by changes in wave refraction patterns generated by a mud bank and has been well-identified on other beaches in this mud-rich region (Anthony and Dolique 2004; Brunier et al. 2016a). As in the case of Yalimapo beach, changes associated with beach rotation and mud-bank welding onto beaches in French Guiana can lead to drastic changes in the availability of beach sand for turtle nesting at temporal scales of years to decades, and can thus have a strong impact on turtle conservation at the regional scale. It is not likely that neighbouring beaches in Suriname (Fig. 1) will serve as nesting sites for turtles faced with diminishing beach space in Yalimapo because Suriname beaches are facing severe pressure from sand extraction that impacts their ecological value (Anthony et al. 2019). The conditions of decreasing beach space availability such as those at Yalimapo are likely to become exacerbated by climate change and sea-level rise (Anthony et al. 2021). These conditions could lead to increasing exposure of nesting sites to flooding and erosion (Fuentes and Abbs 2010; Fuentes-Farias et al. 2010; Varela et al. 2019).
Conclusions
The degradation of nesting habitats is a threat for the offspring of marine turtles. In this context, areas of the beach likely to be an impediment to successful turtle nesting should be identified in order to implement management measures and ensure their better hatching sustainability. The example of Yalimapo beach in French Guiana, subject to the episodic overwhelming presence of mud nefarious to successful nesting, brings out complexities that call for even more rigorous beach monitoring, a condition that also prevails throughout the coastline of the Guianas. Since we cannot accurately predict the coastal dynamics on a seasonal/annual basis, beach management aimed at turtle nesting sustainability becomes complicated. This study has allowed us to identify nest vulnerability and its relationship with beach geomorphological change as an important first step in gaining a better understanding of the dynamics of turtle nesting on a mud-influenced beach in French Guiana, but has also thrown light on the recent dramatic decline in the leatherback turtle population on Yalimapo beach, an emblematic site for turtle nesting in French Guiana which once hosted up to 40% of nesting leatherbacks. The suitable nesting habitat for green turtles on Yalimapo beach has also considerably diminished due to shrinking of the beach and relocation of beach sand eastward in an area unfavourable to turtle nesting. These conditions could be aggravated in the future by climate change effects, including sea-level rise.
Data availability
All data have been included in the manuscript.
References
Abreu-Grobois A, Plotkin PT (2008) Lepidochelys olivacea. IUCN SSC Marine Turtle Specialist Group, Gland, Switzerland. https://doi.org/10.2305/IUCN.UK.2008.RLTS.T11534A3292503.en
Anthony EJ (2019) Beach erosion. In: Finkl C, Makowski C (eds) Encyclopaedia of coastal science. Springer. doi:https://doi.org/10.1007/978-3-319-48657-4_33-2
Anthony EJ, Brondizio ES, dos Santos VF, Gardel A, Besset M (2021) Sustainable management, conservation and restoration of the Amazon delta and Amazon-influenced Guianas coast: a review. Water 13:1371. https://doi.org/10.3390/w13101371
Anthony EJ, Dolique F (2004) The influence of Amazon-derived mud banks on the morphology of sandy, headland-bound beaches in Cayenne, French Guiana: a short- to long-term perspective. Mar Geol 208:249–264. https://doi.org/10.1016/j.margeo.2004.04.011
Anthony E, Brunier G, Gardel A, Hiwat M (2019) Chenier morphodynamics and degradation on the Amazon-influenced coast of Suriname, South America: implications for beach ecosystem services. Front Earth Sci 7:35. https://doi.org/10.3389/feart.2019.00035
Anthony EJ, Gardel A, Gratiot N, Proisy C, Allison MA et al (2010) The Amazon-influenced muddy coast of South America: a review of mud-bank–shoreline interactions. Earth Sci Rev 103:99–121. https://doi.org/10.1016/j.earscirev.2010.09.008
Anthony EJ, Gardel A, Proisy C, Fromard F, Gensac E et al (2013) The role of fluvial sediment supply and river-mouth hydrology in the dynamics of the muddy, Amazon-dominated Amapa-Guianas coast, South America: a three-point research agenda. J South Am Earth Sci 44:18–24. https://doi.org/10.1016/j.jsames.2012.06.005
Baudouin M, de Thoisy B, Chambault P, Berzins R, Entraygues M et al (2015) Identification of key marine areas for conservation based on satellite tracking of post-nesting migrating green turtles (Chelonia mydas). Biol Cons 184:36–41. https://doi.org/10.1016/j.biocon.2014.12.021
Bellard C, Bertelsmeier C, Leadley P, Thuiller W, Courchamp F (2012) Impacts of climate change on the future of biodiversity. Ecol Lett 15:365–377. https://doi.org/10.1111/j.1461-0248.2011.01736.x
Brunier G, Fleury J, Anthony EJ, Gardel A, Dussouillez P (2016a) Close-range airborne Structure-from-Motion Photogrammetry for high-resolution beach morphometric surveys: examples from an embayed rotating beach. Geomorphology 261:76–88. https://doi.org/10.1016/j.geomorph.2016.02.025
Brunier G, Fleury J, Anthony EJ, Pothin V, Vella C, et al. (2016b) Structure-from-Motion photogrammetry for high-resolution coastal and fluvial geomorphic surveys. Géomorphol: Relief, Processus, Environ 22(2):15–25. https://doi.org/10.4000/geomorphologie.11358
Brunier G, Michaud E, Fleury J, Anthony EJ, Morvan S, et al. (2020) Assessing the relationship between macro-faunal burrowing activity and mudflat geomorphology from UAV-based Structure-from-Motion photogrammetry. Remote Sensing of Environment 241:111717. https://doi.org/10.1016/j.rse.2020.111717
Brunier G, Tamura T, Anthony EJ, Dussouillez P, Gardel A (2022) Evolution of the French Guiana coast from Late Pleistocene to Holocene based on chenier and beach sand dating. Reg Environ Change 22:122. https://doi.org/10.1007/s10113-022-01975-3
Bustard HR, Greenham P (1968) Physical and chemical factors affecting hatching in the green sea turtle, Chelonia mydas (L.). Ecology 49:269–276. https://doi.org/10.2307/1934455
Carr A, Carr MH (1972) Site fixity in the Carribean green turtle. Ecology 53:425–429. https://doi.org/10.2307/1934228
Caut S, Guirlet E, Jouquet P, Girondot M (2006a) Influence of nest location and yolkless eggs on hatching success of leatherback turtle nests in French Guiana. Can J Zool 84:908–915. https://doi.org/10.1139/z06-063
Caut S, Hulin V, Girondot M (2006b) Impact of density-dependent nest destruction on emergence success of Guianan leatherback turtles. Anim Conserv 9:189–197. https://doi.org/10.1111/j.1469-1795.2005.00021.x
Chambault P, de Thoisy B, Heerah K, Conchon A, Barrioz S et al (2016a) The influence of oceanographic features on the foraging behavior of the olive ridley sea turtle Lepidochelys olivacea along the Guiana coast. Prog Oceanogr 142:58–71. https://doi.org/10.1016/j.pocean.2016.01.006
Chambault P, de Thoisy B, Kelle L, Berzins R, Bonola M et al (2016b) Inter-nesting behavioural adjustments of green turtles to an estuarine habitat in French Guiana. Mar Ecol Prog Ser 555:235–248. https://doi.org/10.3354/meps11813
Chambault P, Giraudou L, de Thoisy B, Bonola M, Kelle L et al (2017) Habitat use and diving behaviour of gravid olive ridley sea turtles under riverine conditions in French Guiana. J Mar Syst 165:115–123. https://doi.org/10.1016/j.jmarsys.2016.10.005
Chambault P, Pinaud D, Vantrepotte V, Kelle L, Entraygues M et al (2015) Dispersal and diving adjustments of the green turtle Chelonia mydas in response to dynamic environmental conditions during post-nesting migration. PLoS One 10:e0137340. https://doi.org/10.1371/journal.pone.0137340
Chevallier D, Girondot M, Berzins R, Chevalier J, de Thoisy B et al (2020) Survival and pace of reproduction of an endangered sea turtle population, the leatherback Dermochelys coriacea in French Guiana. Endangered Species Res 41:153–165. https://doi.org/10.3354/esr01013
COPERNICUS (2017). Report: European state of the climate. https://climate.copernicus.eu/CopernicusESC. Accessed 10 May 2023
Daniels RC, White TW, Chapman KK (1993) Sea-level rise: destruction of threatened and endangered species habitat in South Carolina. Environ Manage 17:373–385. https://doi.org/10.1007/BF02394680
Engeman RM, Martin RE, Smith HT, Woolard J, Crady CK et al (2006) Impact on predation of sea turtle nests when predator control was removed midway through the nesting season. Wildl Res 33:187–192. https://doi.org/10.1071/WR05049
Fish MR, Cote IM, Gill JA, Jones AP, Renshoff S et al (2005) Predicting the impact of sea-level rise on Caribbean sea turtle nesting habitat. Conserv Biol 19:482–491. https://doi.org/10.1111/j.1523-1739.2005.00146.x
Fretey J, Lescure J (1998) Les tortues marines en Guyane française : bilan de vingt ans de recherche et de conservation. JATBA, Revue D’ethnobiol 40:219–238
Fuentes M, Abbs D (2010) Effects of projected changes in tropical cyclone frequency on sea turtles. Mar Ecol Prog Ser 412:283–292. https://doi.org/10.3354/meps08678
Fuentes-Farias AL, Garduño-Monroy VH, Gutiérrez-Ospina G, Pérez-Cruz L, Meléndez-Herrera E et al (2010) Reconnaissance study of Colola and Maruata, the nesting beaches of marine turtles along the Michoacan coast in southern Mexico. Geofis Int 49:201–212. https://doi.org/10.1016/rgi2010494129
Gardel A, Anthony EJ, Ferreira dos Santos V, Huybrechts N, Lesourd S, et al. (2021) Fluvial sand, Amazon mud, and sediment accommodation in the tropical Maroni River estuary: controls on the transition from estuary to delta and chenier plain. Regional Stud Mar Sci 41:101548. https://doi.org/10.1016/j.rsma.2020.101548
Gardel A, Gratiot N (2005) A satellite image–based method for estimating rates of mud bank migration, French Guiana, South America. J Coastal Res 214:720–728. https://doi.org/10.2112/03-0100.1
Girondot M (2010) Estimating density of animals during migratory waves: application to marine turtles at nesting site. Endangered Species Res 12:85–105. https://doi.org/10.3354/esr00292
Girondot M (2017) Optimizing sampling design to infer marine turtles seasonal nest number for low-and high-density nesting beach using convolution of negative binomial distribution. Ecol Ind 81:83–89. https://doi.org/10.1016/j.ecolind.2017.05.063
Girondot M (2021) HelpersMG: tools for environmental analyses, ecotoxicology and various R functions. 4.6 edn. The Comprehensive R Archive Network. https://CRAN.Rproject.org/package=HelpersMG
Girondot M, Fretey J (1996) Leatherback turtles, Dermochelys coriacea, nesting in French Guiana, 1978–1995. Chelonian Conserv Biol 2:204–208
Girondot M, Rivalan P, Wongsopawiro R, Briane J-P, Hulin V et al (2006) Phenology of marine turtle nesting revealed by a statistical model of the nesting season. BMC Ecol 6:11. https://doi.org/10.1186/1472-6785-6-11
Girondot M, Rizzo A (2015) Bayesian framework to integrate traditional ecological knowledge into ecological modeling: a case study. J Ethnobiol 35:337–353. https://doi.org/10.2993/etbi-35-02-337-353.1
Girondot M, Tucker AD, Rivalan P, Godfrey MH, Chevalier J (2002) Density-dependent nest destruction and population fluctuations of Guianan leatherback turtles. Animal Conservation 5:75–84. https://doi.org/10.1017/S1367943002001099
Hastings WK (1970) Monte Carlo sampling methods using Markov chains and their applications. Biometrika 57:97–109. https://doi.org/10.1093/biomet/57.1.97
Howard R, Bell I, Pike DA (2014) Thermal tolerances of sea turtle embryos: current understanding and future directions. Endangered Species Res 26:75–86. https://doi.org/10.3354/esr00636
Hulin V, Delmas V, Girondot M, Godfrey MH, Guillon J-M (2009) Temperature-dependent sex determination and global change: are some species at greater risk? Oecologia 160:493–506. https://doi.org/10.1007/00442-009-1313-1
IUCN (2021) The IUCN Red List of Threatened Species. Version 2021–1. International Union for Conservation of Nature and Natural Resources, Gland, Suisse. https://www.iucnredlist.org. Accessed 10 May 2023
Jolivet M, Anthony EJ, Gardel A, Brunier G (2019a) Multi-decadal to short-term beach and shoreline mobility in a complex river-mouth environment affected by mud from the Amazon. Front Earth Sci 7:187. https://doi.org/10.3389/feart.2019.00187
Jolivet M, Gardel A, Anthony E (2019b) Multi-decadal changes on the mud-dominated coast of western French Guiana: implications for mesoscale shoreline mobility, river-mouth deflection, and sediment sorting. J Coastal Res 188:185–194. https://doi.org/10.2112/SI88-014.1
Kamel SJ, Mrosovsky N (2004) Nest site selection in leatherbacks, Dermochelys coriacea: individual patterns and their consequences. Anim Behav 58:357–366. https://doi.org/10.1016/j.anbehav.2003.07.021
Kelle L, Gratiot N, Nolibos I, Thérèse J, Wongsopawiro R et al (2007) Monitoring of nesting leatherback turtles (Dermochelys coriacea): contribution of remote sensing for real-time assessment of beach coverage in French Guiana. Chelonian Conserv Biol 6:143–147
Lamont MM, Carthy RR (2007) Response of nesting sea turtles to barrier island dynamics. Chelonian Conserv Biol 6:206–212. https://doi.org/10.2744/1071-8443(2007)6[206:RONSTT]2.0.CO;2
Lamont MM, Houser C (2014) Spatial distribution of loggerhead turtle (Caretta caretta) emergences along a highly dynamic beach in the northern Gulf of Mexico. J Exp Mar Biol Ecol 453:98–107. https://doi.org/10.1016/j.jembe.2013.11.006
Lee J-Y, Marotzke J, Bala G, Cao L, Corti S et al (2021) Future Global Climate: Scenario-Based Projections and Near-Term Information. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Masson-Delmotte, V., P. Zhai, A. Pirani, S.L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M.I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J.B.R. Matthews, T.K. Maycock, T. Waterfield, O. Yelekçi, R. Yu, and B. Zhou (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, pp. 553–672. https://doi.org/10.1017/9781009157896.006
Maurer AS, Seminoff JA, Layman CA, Stapleton SP, Godfrey MH et al (2021) Population viability of sea turtles in the context of global warming. Bioscience. https://doi.org/10.1093/biosci/biab028
Metropolis N, Rosenbluth AW, Rosenbluth MN, Teller AH, Teller E (1953) Equations of state calculations by fast computing machines. J Chem Phys 21:1087–1092. https://doi.org/10.1063/1.1699114
Miller JD (1997) Reproduction in sea turtles. In: Lutz PL, Musick JA (eds) The biology of sea turtles. CRC Press, New York, pp 51–81
Mrosovsky N (1994) Sex ratios of sea-turtles. J Exp Zool 270:16–27. https://doi.org/10.1002/jez.1402700104
Mrosovsky N, Yntema CL (1980) Temperature dependenc of sexual-differentiation in sea turtles -implications for conservation practices. Biol Cons 18:271–280. https://doi.org/10.1016/0006-3207(80)90003-8
Patrício AR, Hawkes LA, Monsinjon JR, Godley BJ, Fuentes MMPB (2021) Climate change and marine turtles: recent advances and future directions. Endang Species Res 44:363–395. https://doi.org/10.3354/esr01110
Péron C (2014) Dynamique littorale et comportement de ponte des tortues marines en Guyane française. Thèse de Doctorat. Université du Littoral Côte d’Opale
Péron C, Chevallier D, Galpin M, Chatelet A, Anthony EJ et al (2013) Beach morphological changes in response to marine turtles nesting: a preliminary study of Awala-Yalimapo beach, French Guiana (South America). J Coastal Res 65:99–104. https://doi.org/10.2112/si65-018.1
Raftery AE, Lewis SM (1992) One long run with diagnostics: implementation strategies for Markov chain Monte Carlo. Stat Sci 7:493–497
Roberts GO, Rosenthal JS (2001) Optimal scaling for various Metropolis-Hastings algorithms. Stat Sci 16:351–367
Rosenthal JS (2011) Optimal proposal distributions and adaptive MCMC. In: Brooks S, Gelman A, Jones G, Meng X-L (eds) MCMC handbook. Chapman and Hall/CRC, pp 93–112. https://doi.org/10.1201/b10905
SantidrianTomillo P, Fonseca L, Paladino FV, Spotila JR, Oro D (2017) Are thermal barriers “higher” in deep sea turtle nests? PLoS One 12:e0177256. https://doi.org/10.1371/journal.pone.0177256
Seminoff JA (2004) Chelonia mydas. IUCN SSC Marine Turtle Specialist Group, Gland, Switzerland. doi:https://doi.org/10.2305/IUCN.UK.2004.RLTS.T4615A11037468.en
Spanier MJ (2010) Beach erosion and nest site selection by the leatherback sea turtle Dermochelys coriacea (Testudines: Dermochelyidae) and implications for management practices at Playa Gandoca, Costa Rica. Rev Biol Trop (Int J Trop Biol) 58:1237–1246. https://doi.org/10.15517/rbt.v58i4.5408
The Second World Ocean Assessment (2021) volume 1. United Nations Publication New York 543. https://www.un.org/regularprocess/sites/www.un.org.regularprocess/files/2011859-e-woa-ii-vol-i.pdf
UICN France and Museum National d’Histoire Naturelle (2017) Chapitres de la Faune vertébrée de Guyane La Liste rouge des espèces menacées en France. UICN France, Paris, p 35 (https://uicn.fr/wp-content/uploads/2017/06/liste-rouge-faune-vertebree-de-guyane.pdf)
Varela MR, Patrício AR, Anderson K, Broderick AC, DeBell L et al (2019) Assessing climate change associated sea level rise impacts on sea turtle nesting beaches using drones, photogrammetry and a novel GPS system. Glob Chang Biol 25(2):753–762. https://doi.org/10.1111/gcb.14526
Wallace BP, DiMatteo AD, Bolten AB, Chaloupka MY, Hutchinson BJ et al (2011) Global conservation priorities for marine turtles. PLoS One 6:e24510. https://doi.org/10.1371/journal.pone.0024510
Weishampel JF, Bagley DA, Ehrhart LM, Rodenbeck BL (2003) Spatiotemporal patterns of annual sea turtle nesting behaviors along an East Central Florida beach. Biol Cons 110:295–303. https://doi.org/10.1016/S0006-3207(02)00232-X
Whitmore CP, Dutton PH (1985) Infertility, embryonic mortality and nest-site selection in leatherback and green sea turtles in Suriname. Biol Cons 34:251–272. https://doi.org/10.1016/0006-3207(85)90095-3
Willis-Norton E, Hazen EL, Fossette S, Shillinger G, Rykaxzewski RR et al (2015) Climate change impacts on leatherback turtle pelagic habitat in the southeast Pacific. Deep Sea Res Part II 113:260–267. https://doi.org/10.1016/j.dsr2.2013.12.019
Witherington B, Hirama S, Mosier A (2011) Sea turtle responses to barriers on their nesting beach. J Exp Mar Biol Ecol 401:1–6. https://doi.org/10.1016/j.jembe.2011.03.012
Acknowledgements
We acknowledge several research grants on the influence of the Amazon on the coast of South America and the ecology of marine turtles. These include funding from the Centre National d’Etudes Spatiales (CNES), the Institut d’Ecologie et de l’Environnement (INEE), and the Université du Littoral Côte d’Opale (ULCO). Some of our data were collected within the CARET-2 program (co-financed by the OP Amazonia and European Union), the ERDF fund, the Fondation de France, the Ministry of Ecology, Sustainable Development, and Energy, the French National Centre for Space Studies (CNES), SHELL, and the French Guiana Regional Council. CP was supported by a PhD scholarship from the Centre National d’Etudes Spatiales (CNES) and the Centre National de la Recherche Scientifique (CNRS). We especially thank all the students and individuals involved in the 5 months of annual fieldwork. The authors also acknowledge the financial support of the ANTIDOT project (Pépinière Interdisciplinaire de Guyane, Mission pour l’Interdisciplinarité, CNRS), the French Guiana Regional Council, the EDF Fondation, and the Fondation de France, the Ministry of Ecology, Sustainable Development, and Energy SHELL. We would also like to thank Anne Corval (CNRS Guyane), Hélène Delvaux (DEAL Guyane), and Eric Hansen (ONCFS DIROM) for their strong support and help in developing this project. We thank Dr. Victoria Grace (www.english-publications.com) for her initial careful reading of the manuscript and correction of the English. The drone flights of CNRS Guyane are carried out in Yalimapo in the framework of the National Observation Service DYNALIT and the French GDR LIGA research network.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Emma Michaud and accepted by Topical Collection Chief Editor Christopher Reyer
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on The highly dynamic French Guiana littoral under Amazon influence: the last decade of multidisciplinary research
Supplementary Information
Below is the link to the electronic supplementary material.
10113_2023_2105_MOESM1_ESM.pdf
Supplementary file1 Nesting season of green turtles in 2012 and 2014. Periods A and B are shown at the top of each graph. (PDF 42 KB)
10113_2023_2105_MOESM2_ESM.pdf
Supplementary file2 Nesting season of leatherbacks in 2012 and 2014. Periods A and B are shown at the top of each graph. (PDF 38 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chevallier, D., Girondot, M., Péron, C. et al. Beach erosion aggravates the drastic decline in marine turtle populations in French Guiana. Reg Environ Change 23, 116 (2023). https://doi.org/10.1007/s10113-023-02105-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10113-023-02105-3