Abstract
Purpose
Laser irradiation activates a range of cellular processes in the periodontal components and promotes tissue repair. However, its effect on osteogenic differentiation of human cementoblast lineage cells remains unclear. This study aimed to examine the effects of high-frequency semiconductor laser irradiation on the osteogenic differentiation of human cementoblast lineage (HCEM) cells.
Methods
HCEM cells were cultured to reach 80% confluence and irradiated with a gallium-aluminum-arsenide (Ga-Al-As) semiconductor laser with a pulse width of 200 ns and wavelength of 910 at a dose of 0–2.0 J/cm2. The outcomes were assessed by analyzing the mRNA levels of alkaline phosphatase (ALP), runt-related transcription factor 2 (RUNX2), and type I collagen (COLL1) using real-time polymerase chain reaction (PCR) analysis 24 h after laser irradiation. Cell mineralization was evaluated using ALP activity, calcium deposition, and Alizarin Red staining.
Results
The laser-irradiated HCEM cells showed significantly enhanced gene expression levels of ALP, RUNX2, and COLL1 as well as ALP activity and calcium concentration in the culture medium compared with the non-irradiated cells. In addition, enhanced calcification deposits were confirmed in the laser-irradiated group compared with the non-irradiated group at 21 and 28 days after the induction of osteogenic differentiation.
Conclusion
High-frequency semiconductor laser irradiation enhances the osteogenic differentiation potential of cultured HCEM cells, underscoring its potential utility for periodontal tissue regeneration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Periodontal tissue consists of the gingiva, alveolar bone, periodontal ligament, and various components of the cementum. The periodontal ligament is a dense fibrous connective tissue located between the cementum and alveolar bone, whose homeostasis is maintained by the expression and function of various cells and intrinsic factors. On the contrary, the cementum is a thin, highly mineralized tissue that covers the root surface of the teeth [1]. The differentiation of cementoblasts and periodontal ligament cells into hard tissue-forming cells plays a crucial role in the repair and regeneration of the periodontium attributed to occlusal trauma or periodontal disease. Recent reports indicate that cementoblasts, unlike periodontal ligament cells, are highly expressed. More specifically, the expression of F-spondin, cementum-derived protein, cementum protein 1, cementum-derived attachment protein, protein-tyrosine phosphatase-like member-a, and miRNA (miR-383, miR-628-5p, and ets variant 1) highlight the fact that cementoblasts could possess unique properties in periodontal repair [2].
Laser is an abbreviation for light amplification by stimulated emission of radiation (LASER). It is a specific electromagnetic wave generated by the inducing radiation of substances in the excited state and has various characteristics according to its wavelength. Therefore, its effectiveness and widespread application in the medical field has been reported.
Photobiomodulation (PBM) or low-level light therapy (LLLT), which provides relatively low-power intravital irradiation with light-emitting diode (LED) or laser light at a specific wavelength of red or near-infrared (NIR) (600–1100 nm), has been shown to induce tissue regeneration, pain relief, and inflammatory modulatory effects [3, 4]. Recently, researchers commonly use the term PBM instead of low-level laser therapy [5, 6]. In addition, PBM has been reportedly used in a variety of dental treatments, oral surgery, oral related pain, and implant dentistry [3, 7, 8]. More specifically, PBM has been found effective in clinical studies as an adjunct to non-surgical periodontal pocket treatment to promote wound healing and relieve pain after periodontal surgical treatment [9, 10]. More recently, high-frequency NIR laser equipment with a high peak power and short pulse duration has been developed, allowing higher accessibility to deep tissues, photochemical activity, and a smaller chance of thermal injury to biological tissues [11]. Moreover, high-frequency semiconductor laser irradiation reduces pain and inflammatory cytokines after tooth extraction and relieves pain caused by bisphosphonate-related osteonecrosis of the jaw [12, 13]. High-frequency semiconductor laser irradiation has also been suggested to relieve pain during orthodontic treatment [14]. Additionally, in vitro studies have demonstrated that high-frequency semiconductor laser irradiation enhances the proliferation and migration of human gingival epithelial cells and mouse calvarial osteoblasts [11, 15]. Furthermore, animal models suggest that high-frequency, low-level semiconductor laser irradiation enhances wound healing in both the soft and hard tissues of tooth extraction sockets, while high-frequency NIR semiconductor laser irradiation leads to metabolic activation of periodontal tissues and increases tooth movement [16, 17]. In addition, high-frequency pulsed semiconductor laser irradiation exhibits biological effects and suppresses bone resorption in a mouse model of periodontitis [18]. Considered together, these findings indicate that semiconductor laser irradiation may affect the regulation of bone metabolism in periodontal cells. However, the efficacy of high-frequency semiconductor lasers for periodontal tissue metabolism in the maxillofacial region is yet to be elucidated.
Therefore, this study aimed to investigate the effects of high-frequency semiconductor laser irradiation on the osteogenic differentiation of the human cementoblast cell line (HCEM), focusing on cells of the cementum with peculiar properties among periodontal tissues.
Materials and methods
Cell culture
Human extracted tooth root surface cementum was immortalized using human telomerase reverse transcriptase after treatment using the sequential enzymatic treatment method devised by Kitagawa et al. [19]. by the experimentation Committee of the Graduate School of Biomedical Science, Hiroshima University. Kitagawa et al., established the HCEM cell line in the department of Oral and Maxillofacial Pathobiology, Graduate School of Biomedical & Health Sciences, Hiroshima University, and provided us the cell line. In the present study, HCEM cell lines 2 was used to conduct the study.
The HCEM cells were cultured in alpha-minimum essential medium (α-MEM) (Sigma Aldrich, St. Louis, MO, USA) containing 10% fetal bovine serum (FBS; Daiichi Chemical, Tokyo, Japan), 60 µg/mL kanamycin (Meiji Seika Pharma, Tokyo, Japan), 250 µg/mL amphotericin B (ICN Biomedicals Corp., Costa Mesa, CA, USA), and 50 U/mL penicillin (Meiji Seika Pharma) at 37℃ and under 5% CO2 at atmospheric pressure.
Laser irradiation
A gallium-aluminum-arsenide (Ga-Al-As) semiconductor laser (Lumix 2, Fisioline s.r.l., Verduno, Italy; main wavelength: 910 nm, maximal power: 45 W, average power: 300 mW, pulse width: 200 ns, maximum pulse repetition rate: 50 kHz, pulse duration: 200 ns, secondary and guiding wavelengths: both 650 nm) was used in the experiment. At the time of laser irradiation, the medium volume of each culture dish was adjusted to the depth of 2 mm from the well floor, and a fixed stand was used so that the height of the irradiation orifice was at the well floor to the position where the guide light covers the bottom of the dish. The height of the orifice was 35 mm for 6-well plates, 25 mm for 12-well plates, and 15 mm for 24-well plates, and laser irradiation was performed to obtain 2.02 J/cm2 energetic quantity per time. The following presets were used: program 1, pulse rate: 30 kHz and overall duty cycle: 0.6%. A VEGA power meter (Ophir Optronics Solutions, Ltd., Jerusalem, Israel) was used to monitor the output. Table 1 lists the descriptions and specifications of the physical parameters of the laser, energy densities, and doses used.
Quantitative real-time polymerase chain reaction (PCR) analysis
HCEM cells were seeded in 6-well plates (FALCON, Flanklin Lakes, NJ, USA) at a density of 1 × 105 cells/well, and the medium was changed once every 2 days [20, 21]. After reaching 80% confluence, the FBS level in the culture medium was set to 0% in a stepwise fashion and laser irradiation at 2.0 J/cm2 was performed. Trizol Isolation Reagent (Thermo Fisher Scientific, Inc., Waltham, MA, USA) was used to recover RNA from the cellular layers 24 h after laser irradiation, and a RNeasy Mini Kit (Qiagen, Hulsterweg, Netherlands) was used to extract total RNA. RNA concentration was determined by measuring the sample absorbance at 260 nm using the NanoDrop One/Onec spectrophotometer (Thermo Fisher Scientific, Inc). ReverTra ace (Toyobo, Osaka, Japan) and Random primer (Toyobo) were used to synthesize cDNA from 1 µg total RNA. The mRNA expression levels of alkaline phosphatase (ALP), type I collagen (COLL1), and runt-related transcription factor (RUNX2) were analyzed using the obtained samples and specific primers (Table 2) and SYBR Green Real-time PCR Master Mix (Toyobo) by the LightCycler System (LightCycler 480 II [Roche Diagnostic, Basel, Switzerland]). The relative mRNA expression levels were analyzed using the ∆∆Ct method and normalized to those of beta-actin (ACTB).
Determination of ALP enzyme activity
The HCEM cells were seeded in 12-well plates (Falcon) at a density of 2 × 10⁴ cells/well containing a-MEM with 10% FBS, which was changed every 2 days [20, 21]. After reaching confluence, the medium was changed to osteogenic differentiation inducing medium (ODM), a-MEM containing 10% FBS supplemented with 0.05 mM ascorbic acid [Sigma Aldrich], 10 mM β-glycerophosphate [Sigma Aldrich], and 100 mM dexamethasone [Sigma Aldrich]). Laser irradiation at 2.0 J/cm2 was performed for every ODM change every 2 days. On the 7th day after osteogenic differentiation was initiated, the cells were washed twice with PBS before adding 0.1% Triton-X-100 and left at room temperature for 10 min before sample extraction. The resulting extract was used in pNPP phosphatase assay kits (Bioassay Systems, Heyward, CA, USA) to determine ALP activity according to the passage method. The absorbance was read at 405 nm using a MultiskanTM FC microplate reader (Thermo Fisher Scientific).
Quantitative determination of the calcium concentration analysis
The HCEM cells were cultured in 12-well plates (Falcon) at a density of 4 × 10⁴ cells/well, and a-MEM containing 10% FBS was changed every 2 days [20, 21]. After reaching 80% confluence, the medium was changed to ODM to induce osteogenic differentiation. The ODM was laser-irradiated at 2.0 J/cm2 once every 2 days. The cells were washed with 10 mM Tris-HCl buffer (pH 7) on the 14th day of osteogenic differentiation and extracted with 10% formic acid at room temperature. The calcium concentration was determined using a calcium E-test Wako (Wako Pure Chemical Industries, Ltd., Osaka, Japan), a colorimetric assay based on the OCPC method, to measure the absorbance of chromogenic products at a wavelength of 620 nm using the Multiskan TMFC microplate reader (Thermo Fisher Scientific).
Assessment of calcified deposits using alizarin red staining
The HCEM cells were cultured in 24-well plates (Falcon) at a density of 2 × 10⁴ cells/well [20, 21], and the medium was changed every 2 days. After reaching 80% confluence, the cells were transferred to the ODM, which was changed once every 2 days, and laser irradiation at 2.0 J/cm2 was performed during every change. The cells were washed with PBS 21 and 28 days following the onset of osteogenic differentiation and fixed with a 37% formaldehyde solution (Wako Pure Chemical Industries, Ltd.) diluted to 4% with purified water. Following this, the cells were treated with 1% Alizarin Red S stain. The stained cells were rinsed several times with UrtraPure TM DNase/RNase-Free Distilled water (Thermo Fisher Scientific) and dried overnight.
Statistical analyses
All the data are presented as mean ± standard deviation. Comparisons between groups were performed using the Mann–Whitney U test. The level of significance was set at * p < 0.05 or ** p < 0.01.
Results
Effects of high-frequency semiconductor laser irradiation on the gene expression levels of ALP, COLL1, and RUNX2 in the HCEM cells
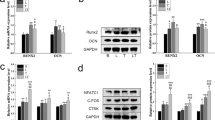
The expression levels of ALP, COLL1, and RUNX2 genes were examined 24 h after the HCEM cells were subjected to laser irradiation. There was a significant surge in ALP gene expression in the laser-irradiated group compared with the non-irradiated group (Fig. 1A). In addition, irradiation at 2 J/cm2 with a high-frequency semiconductor laser produced a near two-fold significant increase in the expression of RUNX2 and COLL1 genes compared with the non-irradiated group (Fig. 1B and C).
Effects of high-frequency semiconductor laser irradiation on gene expression levels of ALP, COLL1, and RUNX2 in HCEM cells. HCEM cells were cultured, and after reaching 80% confluence, FBS was shifted stepwise to 0% and the cells were laser irradiated. Gene expression levels of ALP, COLL1, and RUNX2 were assessed 24 h after laser irradiation. The mRNA expression levels of ALP, COLL1, and RUNX2 were significantly upregulated (P < 0.05) following high-frequency semiconductor laser irradiation (A, B, and C)
Effects of high-frequency semiconductor laser irradiation on mineralization by the HCEM cells
The ALP activity was significantly greater in the laser irradiated HCEM cells compared with the non-irradiated group 7 days after osteogenic differentiation was induced (Fig. 2A). In addition, the Ca concentration in the culture medium was significantly higher in the laser-irradiated group after 14 days of osteogenic differentiation (Fig. 2B). Finally, Alizarin Red staining showing calcified deposits was more noticeable in the laser-irradiated group on the 21st and 28th days of osteogenic differentiation (Fig. 2C).
Effects of high-frequency semiconductor laser irradiation on mineralization by HCEM cells. HCEM cells were cultured and a-MEM containing 10% FBS and the medium was changed every 2 days. After reaching confluence, MEM containing 10% FBS was changed to osteogenic differentiation inducing medium (ODM). Laser irradiation was performed for every ODM change once every 2 days. (A) ALP activity: ALP activity in the HCEM cells treated with laser irradiation was significantly (P < 0.05) higher than that in the untreated control group 7 days after the induction of osteogenic differentiation. (B) Ca2+ concentration: The calcium concentration in HCEM cells was significantly enhanced (P < 0.05) by treatment with laser irradiation compared with that in the non-treated control groups 14 days after the induction of osteogenic differentiation. (C) Alizarin Red staining: HCEM cultures of the whole extracellular matrix region in ODM for 21 and 28 days were stained using Alizarin Red S. The staining level was also enhanced by laser irradiation compared with that in the untreated group
Discussion
The differentiation of osteogenesis-related cells involves the extracellular matrix and includes both collagen and non-collagen components [22]. In particular, ALP is known to be a key player in early-stage osteogenesis, while COLL1 production is considered an early marker of osteoblast differentiation [23, 24]. COLL1 is the main osteogenic process underlying the induction of ALP, and this phenomenon is thought to be the first step of cementoblastic differentiation [23, 25]. Moreover, RUNX2, an osteoblast-specific transcription factor expressed in differentiated osteoblasts and cementoblasts, has been implicated as a major regulator of osteoblast differentiation and gene expression [23, 26]. In addition, RUNX2 has been identified as a key factor in cementoblast and osteoblast differentiation [23]. Therefore, in the present study, we investigated the effects of laser irradiation on the gene expression profiles of ALP, RUNX2, and COLL1. We report a significant enhancement in the gene expression levels of ALP, RUNX2, and COLL1 in HCEM cells receiving 2.0 J/cm2 laser irradiation.
Laser irradiation with a semiconductor laser has been evaluated for effects on the osteogenesis-related gene expression. Studies have shown that visible semiconductor laser wavelengths of 600–700-nm irradiation promote bone differentiation in human-derived osteoblasts and enhance bone differentiation potential in bone marrow-derived mesenchymal stem cells (660 nm, 0.7–4 J/cm2) [8, 27, 28]. Additionally, semiconductor laser radiation (660 nm, 2–4 J/cm2) was found to enhance the expression of bone matrix protein (BMP)-2, osteocalcin (OCN), RUNX2, and ALP in periodontal ligament cells by semiconductor laser irradiation [29]. NIR semiconductor lasers with wavelengths of 700–900 nm have also been found to promote osteoblast (780 nm, 0.25–0.5 W) and bone marrow-derived mesenchymal stem cell osteogenic differentiation (810 nm, 2–6 J/cm2) [30, 31]. Interestingly, higher wavelengths (900–1000 nm) of semiconductor laser irradiation (940 nm, 1–1.5 W/cm2, and 3–4 J) were also found to significantly enhance gene expression of RUNX2, ALP, COLL1, BMP-2, Osterix, and TGFβ in MG-63 human osteoblast-like cells after 24 h [32]. While there are few reports on the effects of high-frequency semiconductor laser irradiation on osteogenic gene expression at a wavelength of 910 nm, its effects are consistent with previous reports.
In the present study, the effect of laser irradiation on the mineralization ability of human cementoblasts was changed to osteogenic differentiation induction medium after reaching confluence. The following effect was verified by examining the levels of ALP and calcium as well as Alizarin Red staining. Our results showed that laser-irradiated HCEM cells had significantly increased ALP concentrations compared with the non-irradiated group 7 days after the initiation of osteogenic differentiation, while the Ca2+ volume in the culture medium was significantly higher in the laser-irradiated group at the 14-day mark. In addition, enhancement of calcified deposits was observed in the laser-irradiated group at 21 and 28 days after bone differentiation was initiated.
To date, the effects of laser irradiation on osteogenic differentiation of cells have been investigated under various conditions. Previous studies have shown that ALP of human osteoblasts is increased by laser irradiation at 632 (0.43 J/cm2) and 660 nm (1.3 J/cm2) wavelengths [28, 33]. Wu et al. reported that 2- and 4-J/cm2 laser irradiation induced osteogenic differentiation of human periodontal ligament cells in a differentiation-inducing medium. Moreover, the study found that laser irradiation had enhanced the mineralization ability of human periodontal ligament cells at 660-nm wavelengths as assessed by osteogenic gene-expression, ALP quantitation, and Alizarin Red stain-based mineralization ability [29]. Additionally, light-emitting diode irradiation of differentiating mesenchymal stem cells with 8 J/cm2 at 660 nm has been shown to significantly increase RUNX2 gene expression, ALP activity, OCN protein concentration, procollagen type I C peptide-protein expression, Ca2+ concentration in culture broth, and calcification deposition assessed by Alizarin Red staining [34]. Additionally, a report on 800–1000-nm semiconductor laser irradiation showed that 905-nm (3.75-J/cm2) laser irradiation increased calcification of murine calvarial-derived osteoblasts [35]. Other studies reported that laser irradiation of human osteoblasts at 830 nm (3 J/cm2) increased ALP activity and highlighted an increase in BMP-2, BMP-4, and ALP gene expression as well as a significant increase in calcified deposits in human dental pump cells after 28 days of culturing following semiconductor laser irradiation at 810 nm (7.6 J/cm2) [36, 37].Regarding the effect of laser irradiation of 4 J/cm2 at 940 nm wavelengths on the osteogenic differentiation of inflamed periodontal ligament stem cells (I-PDLSC), Alizarin Red staining showed no significant changes after 14 and 21 days of induction of osteogenic differentiation. The ALP activity and expression of osteogenesis-related genes reportedly increased significantly after laser irradiation [38].
To date, there has only been one report on the effects of laser irradiation on cementoblasts, in which semiconductor diode laser irradiation was performed at 940 nm (18 J/cm2) on mouse-derived cementoblasts (OCCM-30) seeded on root plates or microplates. The results showed a surge in the expression levels of BMP-2, 3, 6, 7; OCN; BSP; and Von Kossa staining, confirming enhanced mineralization [39]. However, the gene expression levels of RUNX2 and COLL1 did not differ significantly between the laser-irradiated and non-laser-irradiated groups [39]. It is also worth noting that the beneficial effects of laser irradiation on bone metabolism in cells, osteoblasts, and mesenchymal stem cells that comprise the periodontium have been discussed in several recent systematic reviews [18, 40,41,42]. Although the results of this study were similar to those of previous reports, there were differences in several endpoints. More specifically, the large variability of irradiation conditions, such as the type of laser, wavelength, irradiation time, energy quantity, and pulse setting between studies presents a significant barrier to the advancement of basic research on laser irradiation [18, 40,41,42].
The current study demonstrated that 2.0 J/cm2 laser irradiation at a wavelength of 910 nm may enhance the osteogenic differentiation of human cementoblast lineage cells. Nevertheless, its some key limitation has to be acknowledged. First, the laser used in this study was simultaneously irradiated by 650 nm of the guide beam as the sub-wavelength in addition to the dominant wavelength of 910 nm. Therefore, the outcomes of the current report should be interpreted with caution, and comparative examination of the effect of laser irradiation on cell metabolism at each wavelength should be performed in future studies with identical setting conditions. Second, regarding the signaling pathway of laser irradiation, previous basic studies have suggested laser irradiation to be involved in various signaling pathways. However, the signaling pathways and mechanisms associated with PBM have not yet been fully elucidated. Therefore, future studies should investigate the signaling pathway mechanisms of bone differentiation upon radiofrequency laser irradiation in cementoblasts and cells constituting periodontal tissues in more detail. Thirdly, the present study was an in vitro analysis, and the effect in vivo has not been elucidated. For clinical application, in vivo examinations should be carried out in the future, and further examination on the optimum condition of laser irradiation is warranted.
Conclusion
This study revealed that high-frequency semiconductor laser irradiation determines a significant enhancement in the expression levels of osteogenesis-related genes ALP, RUNX2, and COLL1 in HCEM cells and increases the Ca2+ volume in the culture medium and number of calcified deposits, thus enhancing the mineralization ability of cultured human cementoblast lineage cells. This indicates its potential utility for periodontal tissue and cementum regeneration.
Data availability
Not applicable
References
Saygin NE, Giannobile WV, Somerman MJ (2000) Molecular and cell biology of cementum. Periodontol 2000 24:73–98. https://doi.org/10.1034/j.1600-0757.2000.2240105.x
Iwata T, Mizuno N, Nagahara T et al (2021) Identification of regulatory mRNA and microRNA for differentiation into cementoblasts and periodontal ligament cells. J Periodontal Res 56:69–82. https://doi.org/10.1111/jre.12794
Dompe C, Moncrieff L, Matys J et al (2020) Photobiomodulation—underlying mechanism and clinical applications. J Clin Med 9:1–17
Mohamad SA, Milward MR, Hadis MA et al (2021) Photobiomodulation of mineralisation in mesenchymal stem cells. Photochem Photobiol Sci 20:699–714
Hamblin MR (2016) Photobiomodulation or low-level laser therapy. J Biophotonics 9:1122–1124
Anders JJ, Lanzafame RJ, Arany PR (2015) Low-level light/laser therapy versus photobiomodulation therapy. Photomed Laser Surg 33:183–184
Kalhori KAM, Vahdatinia F, Jamalpour MR et al (2019) Photobiomodulation in oral medicine. Photobiomodul Photomed Laser Surg 37:837–861. https://doi.org/10.1089/photob.2019.4706
Fekrazad R, Asefi S, Allahdadi M, Kalhori KAM (2016) Effect of Photobiomodulation on mesenchymal stem cells. Photomed Laser Surg 34:533–542. https://doi.org/10.1089/pho.2015.4029
Zhao H, Hu J, Zhao L (2021) The effect of low-level laser therapy as an adjunct to periodontal surgery in the management of postoperative pain and wound healing: a systematic review and meta-analysis. Lasers Med Sci 36:175–187. https://doi.org/10.1007/s10103-020-03072-5
Ren C, McGrath C, Jin L et al (2017) The effectiveness of low-level laser therapy as an adjunct to non-surgical periodontal treatment: a meta-analysis. J Periodontal Res 52:8–20. https://doi.org/10.1111/jre.12361
Kunimatsu R, Gunji H, Tsuka Y et al (2018) Effects of high-frequency near-infrared diode laser irradiation on the proliferation and migration of mouse calvarial osteoblasts. Lasers Med Sci 33:959–966. https://doi.org/10.1007/s10103-017-2426-0
Mozzati M, Martinasso G, Cocero N et al (2011) Influence of Superpulsed Laser Therapy on healing processes following tooth extraction. Photomed Laser Surg 29:565–571. https://doi.org/10.1089/pho.2010.2921
Romeo U, Galanakis A, Marias C et al (2011) Observation of Pain Control in patients with Bisphosphonate-Induced Osteonecrosis using low level laser therapy: preliminary results. Photomed Laser Surg 29:447–452. https://doi.org/10.1089/pho.2010.2835
Marini I, Gatto MR, Bonetti GA (2010) Effects of superpulsed low-level laser therapy on temporomandibular joint pain. Clin J Pain 26:611–616. https://doi.org/10.1097/AJP.0b013e3181e0190d
Ejiri K, Aoki A, Yamaguchi Y et al (2014) High-frequency low-level diode laser irradiation promotes proliferation and migration of primary cultured human gingival epithelial cells. Lasers Med Sci 29:1339–1347. https://doi.org/10.1007/s10103-013-1292-7
Noda M, Aoki A, Mizutani K et al (2016) High-frequency pulsed low-level diode laser therapy accelerates wound healing of tooth extraction socket: an in vivo study. Lasers Surg Med 48:955–964. https://doi.org/10.1002/lsm.22560
Gunji H, Kunimatsu R, Tsuka Y et al (2018) Effect of high-frequency Near-Infrared Diode Laser Irradiation on Periodontal tissues during experimental tooth Movement in rats. 780:772–780. https://doi.org/10.1002/lsm.22797
Ohsugi Y, Niimi H, Shimohira T et al (2020) In Vitro Cytological responses against laser photobiomodulation for Periodontal Regeneration. Int J Mol Sci 26(9002). https://doi.org/10.3390/ijms21239002
Kitagawa M, Tahara H, Kitagawa S et al (2006) Characterization of established cementoblast-like cell lines from human cementum-lining cells in vitro and in vivo. Bone 39:1035–1042. https://doi.org/10.1016/j.bone.2006.05.022
Kunimatsu R, Yoshimi Y, Hirose N et al (2017) The C-terminus of amelogenin enhances osteogenic differentiation of human cementoblast lineage cells. J Periodontal Res 52:218–224. https://doi.org/10.1111/jre.12384
Kimura A, Kunimatsu R, Yoshimi Y et al (2018) Baicalin promotes osteogenic differentiation of human cementoblast lineage cells Via the Wnt/β Catenin Signaling Pathway. Curr Pharm Des 24:3980–3987. https://doi.org/10.2174/1381612824666181116103514
Zhu J-X, Sasano Y, Takahashi I et al (2001) Temporal and spatial gene expression of Major Bone Extracellular Matrix molecules during embryonic mandibular osteogenesis in rats. Histochem J 33:25–35. https://doi.org/10.1023/A:1017587712914
Inubushi T, Tanaka E, Rego EB et al (2008) Effects of Ultrasound on the proliferation and differentiation of Cementoblast Lineage cells. J Periodontol 79:1984–1990. https://doi.org/10.1902/jop.2008.080081
Franceschi RT, Iyer BS (1992) Relationship between collagen synthesis and expression of the osteoblast phenotype in MC3T3-E1 cells. J Bone Miner Res 7:235–246. https://doi.org/10.1002/jbmr.5650070216
Ishikawa S, Iwasaki K, Komaki M, Ishikawa I (2004) Role of ascorbic acid in Periodontal Ligament Cell differentiation. J Periodontol 75:709–716. https://doi.org/10.1902/jop.2004.75.5.709
Yamaguchi A, Komori T, Suda T (2000) Regulation of osteoblast differentiation mediated by bone morphogenetic proteins, hedgehogs, and Cbfa1. Endocr Rev 21:393–411. https://doi.org/10.1210/edrv.21.4.0403
Stein A, Benayahu D, Maltz L, Oron U (2005) Low-level laser irradiation promotes proliferation and differentiation of human osteoblasts in Vitro. Photomed Laser Surg 23:161–166. https://doi.org/10.1089/pho.2005.23.161
Bloise N, Ceccarelli G, Minzioni P et al (2013) Investigation of low-level laser therapy potentiality on proliferation and differentiation of human osteoblast-like cells in the absence/presence of osteogenic factors. J Biomed Opt 18:128006. https://doi.org/10.1117/1.JBO.18.12.128006
Wu J-Y, Chen C-H, Yeh L-Y et al (2013) Low-power laser irradiation promotes the proliferation and osteogenic differentiation of human periodontal ligament cells via cyclic adenosine monophosphate. Int J Oral Sci 5:85–91. https://doi.org/10.1038/ijos.2013.38
Bayram H, Kenar H, Taşar F, Hasirci V (2013) Effect of low level laser therapy and zoledronate on the viability and ALP activity of Saos-2 cells. Int J Oral Maxillofac Surg 42:140–146. https://doi.org/10.1016/j.ijom.2012.03.026
Soleimani M, Abbasnia E, Fathi M et al (2012) The effects of low-level laser irradiation on differentiation and proliferation of human bone marrow mesenchymal stem cells into neurons and osteoblasts-an in vitro study. Lasers Med Sci 27:423–430. https://doi.org/10.1007/s10103-011-0930-1
Manzano-Moreno FJ, Medina-Huertas R, Ramos-Torrecillas J et al (2015) The effect of low-level diode laser therapy on early differentiation of osteoblast via BMP-2/TGF-β1 and its receptors. J Cranio-Maxillofacial Surg 43:1926–1932. https://doi.org/10.1016/j.jcms.2015.08.026
Stein E, Koehn J, Sutter W et al (2008) Initial effects of low-level laser therapy on growth and differentiation of human osteoblast-like cells. Wien Klin Wochenschr 120:112–117. https://doi.org/10.1007/s00508-008-0932-6
Yamauchi N, Taguchi Y, Kato H, Umeda M (2018) High-power, red-light-emitting diode irradiation enhances proliferation, osteogenic differentiation, and mineralization of human periodontal ligament stem cells via ERK signaling pathway. J Periodontol 89:351–360. https://doi.org/10.1002/JPER.17-0365
Fukuhara E, Goto T, Matayoshi T et al (2006) Optimal low-energy laser irradiation causes temporal G2/M arrest on rat calvarial osteoblasts. Calcif Tissue Int 79:443–450. https://doi.org/10.1007/s00223-006-0072-9
Khadra M, Lyngstadaas SP, Haanæs HR, Mustafa K (2005) Effect of laser therapy on attachment, proliferation and differentiation of human osteoblast-like cells cultured on titanium implant material. Biomaterials 26:3503–3509. https://doi.org/10.1016/j.biomaterials.2004.09.033
Matsui S, Tsujimoto Y, Matsushima K (2007) Stimulatory effects of Hydroxyl Radical Generation by Ga-Al-As laser irradiation on mineralization ability of Human Dental Pulp cells. Biol Pharm Bull 30:27–31. https://doi.org/10.1248/bpb.30.27
Gholami L, Hendi SS, Saidijam M et al (2022) Near-infrared 940-nm diode laser photobiomodulation of inflamed periodontal ligament stem cells. Lasers Med Sci 37:449–459. https://doi.org/10.1007/s10103-021-03282-5
Bozkurt SB, Hakki EE, Kayis SA et al (2017) Biostimulation with diode laser positively regulates cementoblast functions, in vitro. Lasers Med Sci 32:911–919. https://doi.org/10.1007/s10103-017-2192-z
Garzón J, Baldion PA, Grajales M, Escobar LM (2022) Response of osteoblastic cells to low-level laser treatment: a systematic review. Lasers Med Sci 37:3031–3049. https://doi.org/10.2174/1574888X17666220527090321
Mylona V, Anagnostaki E, Chiniforush N et al (2022) Photobiomodulation effects on periodontal ligament stem cells: a systematic review of in-vitro studies. Curr Stem Cell Res Ther. https://doi.org/10.2174/1574888X17666220527090321
Lopes C, de CA, Limirio JPJO, Zanatta LSA et al (2022) Effectiveness of Photobiomodulation Therapy on Human Bone Healing in Dentistry: a systematic review. Photobiomodul Photomed Laser Surg 40:440–453. https://doi.org/10.1089/photob.2021.0092
Acknowledgements
This work was supported by the Grants-in-Aid for Scientific Research [grant number 19K10385, 23K16203 and 24K20081] from the Japan Society for the Promotion of Science.
Funding
This work was supported by the Grants-in-Aid for Scientific Research [grant number 19K10385, 23K16203 and 24K20081] from the Japan Society for the Promotion of Science.
Open Access funding provided by Hiroshima University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article..
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nakatani, A., Kunimatsu, R., Sakata, S. et al. High-frequency low-intensity semiconductor laser irradiation enhances osteogenic differentiation of human cementoblast lineage cells. Lasers Med Sci 39, 174 (2024). https://doi.org/10.1007/s10103-024-04127-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10103-024-04127-7