Abstract
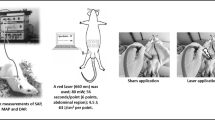
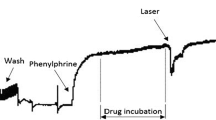
We found several studies that have used the aortic rings as an experimental model, mainly for the testing of new drugs or new therapies that try to reverse or prevent endothelial dysfunction or characterize its mechanism of action in a biological system, creating the knowledge necessary to obtain the treatment of those several diseases, where many of these treatments involve photobiomodulation therapies. We also found numerous wavelengths represented by different colors of LASER or LED in which frequently, the mechanism of action in biological systems is unknown. This study has as main objective to investigate the effects of the Violet LED Light (405 nm) by using isolated aortic rings, looking for nitric oxide (NO) release, and evaluating if Violet LED Light can modulate the superoxide dismutase (SOD) activity. We performed a vascular reactivity study in isolated aortic rings from normotensive rats with a single LED application. Besides it, the rings were pre-incubated with soluble guanylate cyclase (sGC) inhibitor or endothelial NO synthase inhibitor and subsequently underwent the application of the Violet LED. The cell viability and nitric oxide release in cell culture of human umbilical codon vein cells (HUVEC) were analyzed. In the vascular reactivity experiment, we observed a peak of vasodilation when applying light to the aortic rings. The soluble guanylate cyclase inhibitor abolished the relaxation induced by the Violet LED Light. However, the NO synthase inhibitor did not modify the Violet LED effect. In an isolated system, we verified that the Violet LED Light can increase SOD activity. Our results suggest that Violet LED Light induces vasodilation by a mechanism dependent on sGC activation, and not by NOS activation, and part of this effect could be due to the increase of SOD activity.





Similar content being viewed by others
References
Oishi JC, Castro CA, Silva KA, Fabricio V, Cárnio EC, Phillips SA, Duarte ACGO, Rodrigues GJ (2018) Endothelial dysfunction and inflammation precedes elevations in blood pressure induced by a high-fat diet. Arq Bras Cardiol 110(6):558–567. https://doi.org/10.5935/abc.20180086 Erratum in: Arq Bras Cardiol. 2019 Jan;112(1):116. PMID: 30226915; PMCID: PMC6023639
Ankri R, Friedman H, Savion N, Kotev-Emeth S, Breitbart H, Lubart R (2010 Apr) Visible light induces nitric oxide (NO) formation in sperm and endothelial cells. Lasers Surg Med 42(4):348–352. https://doi.org/10.1002/lsm.20849
Chen X, Buerk DG, Barbee KA, Jaron D (2007) A model of NO/O2 transport in capillary-perfused tissue containing an arteriole and venule pair. Ann Biomed Eng 35(4):517–529. https://doi.org/10.1007/s10439-006-9236-z Epub 2007 Jan 19
Regina A, Carollo H, Desenvolvimento de nanodispersões de fase líquido-cristalina para a liberação cutânea da associação de complexo nitrosilo de rutênio e protoporfirina IX na terapia fotodinâmica do câncer de pele Desenvolvimento de nanodispersões de fase líquido-cristalina, (2011).
Moncada S, Palmer RM, Higgs EA (1989 Jun 1) Biosynthesis of nitric oxide from L-arginine. A pathway for the regulation of cell function and communication. Biochem Pharmacol 38(11):1709–1715. https://doi.org/10.1016/0006-2952(89)90403-6
Rapoport RM, Draznin MB, Murad F (1983) Endothelium-dependent relaxation in rat aorta may be mediated through cyclic GMP-dependent protein phosphorylation. Nature 306(5939):174–176. https://doi.org/10.1038/306174a0
Napoli C, Paolisso G, Casamassimi A, Al-Omran M, Barbieri M, Sommese L, Infante T, Ignarro LJ (2013) Effects of nitric oxide on cell proliferation: novel insights. J Am Coll Cardiol 62(2):89–95. https://doi.org/10.1016/j.jacc.2013.03.070 Epub 2013 May 9
Oh KJ, Park J, Lee HS, Park K (2018) Effects of light-emitting diodes irradiation on human vascular endothelial cells. Int J Impot Res 30(6):312–317. https://doi.org/10.1038/s41443-018-0051-5 Epub 2018 Jul 25
Opländer C, Deck A, Volkmar CM, Kirsch M, Liebmann J, Born M, van Abeelen F, van Faassen EE, Kröncke KD, Windolf J, Suschek CV (2013) Mechanism and biological relevance of blue-light (420-453 nm)-induced nonenzymatic nitric oxide generation from photolabile nitric oxide derivates in human skin in vitro and in vivo. Free Radic Biol Med 65:1363–1377. https://doi.org/10.1016/j.freeradbiomed.2013.09.022 Epub 2013 Oct 9
Rohringer S, Holnthoner W, Chaudary S, Slezak P, Priglinger E, Strassl M, Pill K, Mühleder S, Redl H, Dungel P (2017) The impact of wavelengths of LED light-therapy on endothelial cells. Sci Rep 7(1):10700. https://doi.org/10.1038/s41598-017-11061-y PMID: 28878330; PMCID: PMC5587748
Fattman CL, Schaefer LM, Oury TD (2003) Extracellular superoxide dismutase in biology and medicine. Free Radic Biol Med 35(3):236–256. https://doi.org/10.1016/s0891-5849(03)00275-2
Miao L, St Clair DK (2009) Regulation of superoxide dismutase genes: implications in disease. Free Radic Biol Med 47(4):344–356. https://doi.org/10.1016/j.freeradbiomed.2009.05.018 Epub 2009 May 25. PMID: 19477268; PMCID: PMC2731574
Yamakura F, Kawasaki H (2010) Post-translational modifications of superoxide dismutase. Biochim Biophys Acta 1804(2):318–325. https://doi.org/10.1016/j.bbapap.2009.10.010 Epub 2009 Oct 22
Zelko IN, Mariani TJ, Folz RJ (2002) Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med 33(3):337–349. https://doi.org/10.1016/s0891-5849(02)00905-x
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65(1-2):55–63. https://doi.org/10.1016/0022-1759(83)90303-4
Thomas DD, Ridnour LA, Espey MG, Donzelli S, Ambs S, Hussain SP, Harris CC, DeGraff W, Roberts DD, Mitchell JB, Wink DA (2006) Superoxide fluxes limit nitric oxide-induced signaling. J Biol Chem. 281(36):25984–25993. https://doi.org/10.1074/jbc.M602242200 Epub 2006 Jul 7
Haslam RJ, McClenaghan MD (1974) Effects of collagen and of aspirin on the concentration of guanosine 3':5'-cyclic monophosphate in human blood platelets: measurement by a prelabelling technique. Biochem J 138(2):317–320. https://doi.org/10.1042/bj1380317 PMID: 4362746; PMCID: PMC1166211
Haslam RJ, Davidson MM, Davies T, Lynham JA, McClenaghan MD (1978) Regulation of blood platelet function by cyclic nucleotides. Adv Cyclic Nucleotide Res 9:533–552
Chiang TM, Dixit SN, Kang AH (1976 Aug) Effect of cyclic 3',5'-guanosine monophosphate on human platelet function. J Lab Clin Med 88(2):215–221
Moro MA, Russel RJ, Cellek S, Lizasoain I, Su Y, Darley-Usmar VM, Radomski MW, Moncada S (1996) cGMP mediates the vascular and platelet actions of nitric oxide: confirmation using an inhibitor of the soluble guanylyl cyclase. Proc Natl Acad Sci U S A 93(4):1480–1485. https://doi.org/10.1073/pnas.93.4.1480 PMID: 8643658; PMCID: PMC39965
Mergia E, Friebe A, Dangel O, Russwurm M, Koesling D (2006) Spare guanylyl cyclase NO receptors ensure high NO sensitivity in the vascular system. J Clin Invest 116(6):1731–1737. https://doi.org/10.1172/JCI27657 Epub 2006 May 4. PMID: 16614755; PMCID: PMC1435723
Friebe A, Mergia E, Dangel O, Lange A, Koesling D (2007) Fatal gastrointestinal obstruction and hypertension in mice lacking nitric oxide-sensitive guanylyl cyclase. Proc Natl Acad Sci U S A 104(18):7699–7704. https://doi.org/10.1073/pnas.0609778104 Epub 2007 Apr 23. PMID: 17452643; PMCID: PMC1863512
Dangel O, Mergia E, Karlisch K, Groneberg D, Koesling D, Friebe A (2010) Nitric oxide-sensitive guanylyl cyclase is the only nitric oxide receptor mediating platelet inhibition. J Thromb Haemost 8(6):1343–1352. https://doi.org/10.1111/j.1538-7836.2010.03806.x Epub 2010 Feb 11
Roger S, Paysant J, Badier-Commander C, Cordi A, Verbeuren TJ, Félétou M (2010) Anti-aggregating effect of BAY 58-2667, an activator of soluble guanylyl cyclase. Vascul Pharmacol 53(5-6):281–287. https://doi.org/10.1016/j.vph.2010.09.008 Epub 2010 Oct 7
Tamargo J, Duarte J, Caballero R, Delpón E (2010 Sep) Cinaciguat, a soluble guanylate cyclase activator for the potential treatment of acute heart failure. Curr Opin Investig Drugs 11(9):1039–1047
Isenberg JS, Ridnour LA, Perruccio EM, Espey MG, Wink DA, Roberts DD (2005) Thrombospondin-1 inhibits endothelial cell responses to nitric oxide in a cGMP-dependent manner. Proc Natl Acad Sci U S A 102(37):13141–13146. https://doi.org/10.1073/pnas.0502977102 Epub 2005 Sep 6. PMID: 16150726; PMCID: PMC1201579
Nakamura T, Igarashi R, Kurashina T, Saito Y, Hoshino J, Sumino H, Sakamoto H, Nagai R (1999) Lecithinized superoxide dismutase induces vasodilation; evidence of direct contribution of superoxide anions to modulating vascular tone. Life Sci 64(4):PL65–PL70. https://doi.org/10.1016/s0024-3205(98)00564-5
Rodrigues GJ, Lunardi CN, Lima RG, Santos CX, Laurindo FR, da Silva RS, Bendhack LM (2008) Vitamin C improves the effect of a new nitric oxide donor on the vascular smooth muscle from renal hypertensive rats. Nitric Oxide 18(3):176–183. https://doi.org/10.1016/j.niox.2007.12.002 Epub 2007 Dec 25
Acknowledgements
Credits to DMC Equipment LTDA for providing the Olympus® Elite equipment.
Author information
Authors and Affiliations
Contributions
LM and GR: Conceptualization; LM and GR: Data curation; LM, MM, LL, and GR: Formal analysis; GR: Funding acquisition; LM: Investigation; GR: Methodology; GR: Project administration; LM: Resources; GR: Supervision; GR: Validation; GR: Visualization; LM: Roles/Writing—original draft; LM: Writing—review & editing
Corresponding author
Ethics declarations
Ethical approval
This study received a favorable opinion from the Animal Use Ethics Committee (CEUA) of the Federal University of São Carlos, under the Protocol number: 6382051216.
Conflict of interest
The authors declare no competing interests.
Additional information
Highlights
Violet led induces vasorelaxation by nitric oxide–dependent sGC activation and non-dependent of endothelium and NOS activation. It is also able to increase SOD activity.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 70 kb)
Rights and permissions
About this article
Cite this article
de Moraes, L.H.O., Mancini, M.W., Almeida-Lopes, L. et al. Violet LED induces vasodilation in rat aortic rings by soluble guanylate cyclase–dependent mechanism and increases SOD activity. Lasers Med Sci 37, 537–544 (2022). https://doi.org/10.1007/s10103-021-03293-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-021-03293-2