Abstract
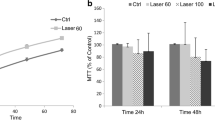
The aim of the present study was to investigate the influence of low-level red (660 nm) and infrared (780 nm) laser with four different radiance exposures on human umbilical vein endothelial cells (HUVECs) in vitro. HUVECs (1.5 × 104) were incubated in 96-well culture plates. The cells were maintained in M199 medium supplemented with 20% fetal bovine serum, 1% antibiotic (penicillin), 1% anti-mycotic (Fungizone), and 1% endothelial cell growth supplement. After centrifugation, irradiations (660/780 nm, 40 mW, 1, 5, 10, and 20 J/cm2, 1 s, 5 s, 10 s, and 20 s, respectively, total energy 0.4 J, 2 J, 4 J, and 8 J, and beam spot size at target 0.04 cm2) were performed at the bottom of Falcon tubes such that the laser beam directly reached the cell without passing through the culture medium. The cells were divided into groups based on radiant exposures. Cell viability and protein concentration were verified after 1, 2, 3, 6, 8, and 10 days. Red laser increased the cell viability and protein concentration in all groups (three-way ANOVA, p < 0.05) beginning on the second day. The greatest peak compared with the control was found when the radiant exposure was 5 J/cm2 and 10 J/cm2. Infrared laser inhibited cell viability and modulated the protein concentration in the cells, with the highest peak protein concentration found on the second day in the group with radiant exposure of 1 J/cm2 and 10 J/cm2 (three-way ANOVA, p < 0.05). Red laser increased the viability and concentration of total proteins in HUVECs, whereas infrared laser had an inhibitory effect on cell viability, while maintaining the total protein concentration similar to that found in the control group.




Similar content being viewed by others
References
Szymanska J, Goralczyk K, Klawe JJ, Lukowicz M, Michalska M, Goralczyk B, Zalewski P, Newton JL, Gryko L, Zajac A, Rosc D (2013) Phototherapy with low-level laser influences the proliferation of endothelial cells and vascular endothelial growth factor and transforming growth factor-beta secretion. J Physiol Pharmacol 3:387–391
Atkinson BT, Jasuja R, Chen VM, Nandivada P, Furie B, Furie BC (2010) Laser - induced endothelial cell activation supports fibrin formation. Blood 22:4675–4683
Patan S (2004) Vasculogenesis and angiogenesis. Cancer Treat Res 117:3–32
Gasparyan LV, Brill G, Makela AM (2005) Activation of angiogenesis under influence of red low-level laser radiation. Proc. SPIE 5968, laser Florence. https://doi.org/10.1117/12.660039
Crabtree B, Subramanian V (2007) Behavior of endothelial cells on Matrigel and development of a method for a rapid and reproducible in vitro angiogenesis assay. In Vitro Cell Dev Biol Anim 43:87. https://doi.org/10.1007/s11626-007-9012-x
Vailhé B, Villet D, Feige JJ (2001) In vitro models of vaculogenesis and angiogenesis. Lab Investig 81(4):439–452
Schmidt M, Paes K, De Mazière A, Smyczek T, Yang S, Gray A, French D, Kasman I, Klumperman J, Rice DS, Ye W (2013) EGFL7 regulates the collective migration of endothelial cells by restricting their spatial distribution. Development 134:2913–2923. https://doi.org/10.1242/dev.002576
Morin KT, Tranquilo RT (2013) In vitro models of angiogenesis and vasculogenesis in fibrin gel. Exp Cell Res 319:2409–2417
Alghamdi KM, Kumar A, Moussa NA (2012) Low-level laser therapy: a useful technique for enhancing the proliferation of various cultured cells. Lasers Med Sci 27(1):237–249
Gargett CE, Bucak K, Rogers PAW (2000) Isolation, characterization and long-term culture of human myometrial microvascular endothelial cells. Hum Reprod 15(2):293–301
Terena SML, Fernandes KPS, Bussadori SK, Junior AB, da Silva DFT, Magalhães EMR, Mesquita Ferrari RA (2018) Infrared laser improves collagen organization in muscle and tendon tissue during the process of compensatory overload. Photomed Laser Surg. https://doi.org/10.1089/pho.2017.4302
Baptista PT, Martins MD, Pavesi VCS, Bussadori SK, Fernandes KPS, Júnior DSP, Mesquita Ferrari RA (2011) Influence of laser photobiomodulation on collagen IV during skeletal muscle tissue remodeling after injury in rats. Photomed Laser Surg 29(1):11–17
Moore P, Ridgway TD, Higbee RG, Howard EW, Lucroy MD (2005) Effect of wavelength on low-intensity laser irradiation-stimulated cell proliferation in vitro. Lasers Surg Med 36:8–12
Fujihara NA, Hiraki KRN, Marques MM (2006) Irradiation at 780nm increases proliferation rate of osteoblasts independently of dexamethasone presence. Lasers Surg Med 38:332–336
Terena SML, Fernandes KPS; Bussadori SK, Alves AN, Mesquita Ferrari RA (2015) Effects of low -level laser in the morphology of the skeletal muscle fiber during compensatory hypertrophy in plantar muscle of rats. Proceedings of SPIE - International Society for Optical Engineering. https://doi.org/10.1117/12.2181034
Teixeira Silva DF, Mesquita Ferrari RA, Fernandes KPS, Raele MP, Wetter NU, Deana AM (2012) Effective transmission of light for media culture, plates and tubes. Photochem Photobiol 88(5):1211–1216
Gao X, Xing D (2009) Molecular mechanisms of cell proliferation induced by low power laser irradiation. J Biomed Sci 16(4):1–16
Chen CH, Hung HS, Hsu SH (2006) Low-energy laser irradiation increases endothelial cell proliferation, migration, and eNOS gene expression possibility via PI3K signal pathway. Lasers Surg Med 40:46–54
Stoddart MJ (2011) Cell viability assays: introduction. In: Stoddart M (eds) Mammalian cell viability. Methods Mol Biol 740:1–6. https://doi.org/10.1007/978-1-61779-108-6_1
Redmile – Gordon MA, Armenise E, White RP, Goulding KW (2013) A comparison of two colorimetric assays, based upon Lowry and Bradford techniques, to estimate total protein in soil extracts. Soil Biol Biochem 67(100):166–173
Cury V, Moretti AIS, Assis L, Bossini P, Crusca JS, Neto CB, Fangel R, De Souza HP, Hamblim MR, Parizzoto NA (2013) Low level laser therapy increases angiogenesis ina model of ischemic skin flap in rats mediated by VEGF, HIF - 1〈 and MMp-2. J Photochem Photobiol 125:164–170. https://doi.org/10.1016/j.jphotobiol.2013.06.004
Lukowicz M, Szymanska J, Goràlczyk K, Zajac A, Rosc D (2013) Effect of low-level laser therapy and high intensity laser therapy on endothelial cell proliferation in vitro - preliminary communication. Proc SPIE. https://doi.org/10.1117/12.2012658
Medeiros ML, Araújo – Filho I, Da Silva EMN, Queiroz WSS, Soares CD, De Carvalho MGF, Maciel MAM (2016) Effect of low-level laser therapy on angiogenesis and matrix metalloproteinase - 2 immunoexpression in wound repair. Lasers Med Sci. https://doi.org/10.1007/s10103-016-2080-y
Schindl AS, Merwald H, Schindl LS, Kaun C, Wojta J (2003) Direct stimulatory effect of low intensity 670nm laser irradiation on human endothelial cell proliferation. Br J Dermatol 148:334–336
Góralczyk K, Szymanska J, Drela E, Kotzbach R, Dubiel M, Michalska M, Góralczyk B, Zajac A, Rosc D (2015) Effect of LLLT on endothelial cells culture. Lasers Med Sci 30:273–278
Góralczyk K, Szymanska J, Szot K, Fisz J, Rosc D (2016) Low-level laser irradiation effect on endothelial cells under conditions of hyperglycemia. Lasers Med Sci. https://doi.org/10.1007/s10103-016-1880-4
Funding
This work was supported by UNINOVE and the Brazilian fostering agency Coordination for the Advancement of Higher Education Personnel- CAPES (www.capes.gov.br; process number 014704/2013-07 SMLT).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This study received approval from Ethics Committee on Animal Research of University Nove de Julho (process number AN007/2013).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Terena, S.M.L., Mesquita-Ferrari, R.A., de Siqueira Araújo, A.M. et al. Photobiomodulation alters the viability of HUVECs cells. Lasers Med Sci 36, 83–90 (2021). https://doi.org/10.1007/s10103-020-03016-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-020-03016-z