Abstract
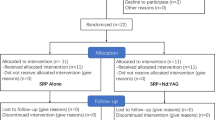
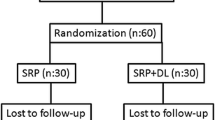
Bactericidal and detoxification effects of diode laser (DL) have been reported in periodontal treatment. The objective of this study was investigating the additional effect of DL with nonsurgical periodontal treatment on the red complex bacteria in type 2 diabetes mellitus (DM) patients with chronic periodontitis (CP). Sixty type 2 DM patients with chronic periodontitis (CP) were randomly assigned in two parallel groups to receive scaling root planning (SRP, n = 30) or SRP followed by DL periodontal pocket irradiation (SRP + DL, n = 30). Recording of clinical parameters and subgingival plaque sampling were performed at baseline, and post therapy (1 and 3 months after treatment). Amounts of Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia were evaluated with quantitative RT-PCR. Significant reductions for numbers of all three bacterial species were observed at 1 and 3 months compared with baseline for both treatments (p < 0.001), but no significant differences were found between two groups regarding bacterial reductions at these follow-up time points. No additional benefit of DL as an adjunct to nonsurgical periodontal therapy was recognized in the reduction of P. gingivalis, T. denticola, and T. forsythia for type 2 DM patients with CP. Further studies are required to clarify the effects of diode laser on the other periodontopathogens.

Similar content being viewed by others
References
Kidambi S, Patel SB (2008) Diabetes mellitus: considerations for dentistry. J Am Dent Assoc 139(Suppl):8S–18S
O’Connell PA, Taba M, Nomizo A et al (2008) Effects of periodontal therapy on glycemic control and inflammatory markers. J Periodontol 79(5):774–783
Loe H (1993) Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care 16(1):329–334
Verhulst MJL, Loos BG, Gerdes VEA, Teeuw WJ (2019) Evaluating all potential oral complications of diabetes mssellitus. Front Endocrinol (Lausanne) 10:56
Bartold PM, Van Dyke TE (2017) Host modulation: controlling the inflammation to control the infection. Periodontol 75(1):317–329
Sgolastra F, Severino M, Pietropaoli D, Gatto R, Monaco A (2013) Effectiveness of periodontal treatment to improve metabolic control in patients with chronic periodontitis and type 2 diabetes: a meta-analysis of randomized clinical trials. J Periodontol 84(7):958–973
Preshaw PM, Alba AL, Herrera D et al (2012) Periodontitis and diabetes: a two-way relationship. Diabetologia. 55(1):21–31
Mealey BL, Oates TW (2006) American Academy of P. Diabetes mellitus and periodontal diseases. J Periodontol 77(8):1289–1303
Manouchehr-Pour M, Spagnuolo PJ, Rodman HM, Bissada NF (1981) Comparison of neutrophil chemotactic response in diabetic patients with mild and severe periodontal disease. J Periodontol 52(8):410–415
McMullen JA, Van Dyke TE, Horoszewicz HU, Genco RJ (1981) Neutrophil chemotaxis in individuals with advanced periodontal disease and a genetic predisposition to diabetes mellitus. J Periodontol 52(4):167–173
Taylor JJ, Preshaw PM, Lalla E (2013) A review of the evidence for pathogenic mechanisms that may link periodontitis and diabetes. J Periodontol 84(4 Suppl):S113–S134
Mealey BL, Moritz AJ (2003) Hormonal influences: effects of diabetes mellitus and endogenous female sex steroid hormones on the periodontium. Periodontol 32:59–81
Bascones-Martinez A, Matesanz-Perez P, Escribano-Bermejo M, Gonzalez-Moles MA, Bascones-Ilundain J, Meurman JH (2011) Periodontal disease and diabetes-review of the literature. Med Oral Patol Oral Cir Bucal 16(6):e722–e729
Schmidt AM, Weidman E, Lalla E et al (1996) Advanced glycation endproducts (AGEs) induce oxidant stress in the gingiva: a potential mechanism underlying accelerated periodontal disease associated with diabetes. J Periodontal Res 31(7):508–515
Kornman KS, Page RC, Tonetti MS (1997) The host response to the microbial challenge in periodontitis: assembling the players. Periodontol 14:33–53
Hotamisligil GS (2000) Molecular mechanisms of insulin resistance and the role of the adipocyte. Int J Obes Relat Metab Disord 24(Suppl 4):S23–S27
Rotter V, Nagaev I, Smith U (2003) Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J Biol Chem 278(46):45777–45784
Nishimura F, Murayama Y (2001) Periodontal inflammation and insulin resistance--lessons from obesity. J Dent Res 80(8):1690–1694
Taylor GW, Burt BA, Becker MP et al (1996) Severe periodontitis and risk for poor glycemic control in patients with non-insulin-dependent diabetes mellitus. J Periodontol 67(10 Suppl):1085–1093
Makiura N, Ojima M, Kou Y et al (2008) Relationship of Porphyromonas gingivalis with glycemic level in patients with type 2 diabetes following periodontal treatment. Oral Microbiol Immunol 23(4):348–351
Tervonen T, Oliver RC, Wolff LF, Bereuter J, Anderson L, Aeppli DM (1994) Prevalence of periodontal pathogens with varying metabolic control of diabetes mellitus. J Clin Periodontol 21(6):375–379
Sbordone L, Ramaglia L, Barone A, Ciaglia RN, Tenore A, Iacono VJ (1995) Periodontal status and selected cultivable anaerobic microflora of insulin-dependent juvenile diabetics. J Periodontol 66(6):452–461
Yuan K, Chang CJ, Hsu PC, Sun HS, Tseng CC, Wang JR (2001) Detection of putative periodontal pathogens in non-insulin-dependent diabetes mellitus and non-diabetes mellitus by polymerase chain reaction. J Periodontal Res 36(1):18–24
Hintao J, Teanpaisan R, Chongsuvivatwong V, Ratarasan C, Dahlen G (2007) The microbiological profiles of saliva, supragingival and subgingival plaque and dental caries in adults with and without type 2 diabetes mellitus. Oral Microbiol Immunol 22(3):175–181
Campus G, Salem A, Uzzau S, Baldoni E, Tonolo G (2005) Diabetes and periodontal disease: a case-control study. J Periodontol 76(3):418–425
Ebersole JL, Holt SC, Hansard R, Novak MJ (2008) Microbiologic and immunologic characteristics of periodontal disease in Hispanic Americans with type 2 diabetes. J Periodontol 79(4):637–646
Holt SC, Ebersole JL (2005) Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: the “red complex”, a prototype polybacterial pathogenic consortium in periodontitis. Periodontol 38:72–122
da Cruz GA, de Toledo S, Sallum EA et al (2008) Clinical and laboratory evaluations of non-surgical periodontal treatment in subjects with diabetes mellitus. J Periodontol 79(7):1150–1157
Aoki A, Sasaki KM, Watanabe H, Ishikawa I (2004) Lasers in nonsurgical periodontal therapy. Periodontol 36:59–97
Harris DM, Yessik M (2004) Therapeutic ratio quantifies laser antisepsis: ablation of Porphyromonas gingivalis with dental lasers. Lasers Surg Med 35(3):206–213
Moritz A, Gutknecht N, Doertbudak O et al (1997) Bacterial reduction in periodontal pockets through irradiation with a diode laser: a pilot study. J Clin Laser Med Surg 15(1):33–37
Moritz A, Schoop U, Goharkhay K et al (1998) Treatment of periodontal pockets with a diode laser. Lasers Surg Med 22(5):302–311
Kamma JJ, Vasdekis VG, Romanos GE (2009) The effect of diode laser (980 nm) treatment on aggressive periodontitis: evaluation of microbial and clinical parameters. Photomed Laser Surg 27(1):11–19
Caruso U, Nastri L, Piccolomini R, d’Ercole S, Mazza C, Guida L (2008) Use of diode laser 980 nm as adjunctive therapy in the treatment of chronic periodontitis. A randomized controlled clinical trial. New Microbiol 31(4):513–518
De Micheli G, de Andrade AK, Alves VT, Seto M, Pannuti CM, Cai S (2011) Efficacy of high intensity diode laser as an adjunct to non-surgical periodontal treatment: a randomized controlled trial. Lasers Med Sci 26(1):43–48
Euzebio Alves VT, de Andrade AK, Toaliar JM et al (2013) Clinical and microbiological evaluation of high intensity diode laser adjutant to non-surgical periodontal treatment: a 6-month clinical trial. Clin Oral Investig 17(1):87–95
Kocak E, Saglam M, Kayis SA et al (2016) Nonsurgical periodontal therapy with/without diode laser modulates metabolic control of type 2 diabetics with periodontitis: a randomized clinical trial. Lasers Med Sci 31(2):343–353
Giannelli M, Formigli L, Lorenzini L, Bani D (2012) Combined photoablative and photodynamic diode laser therapy as an adjunct to non-surgical periodontal treatment: a randomized split-mouth clinical trial. J Clin Periodontol 39(10):962–970
Ustun K, Erciyas K, Sezer U et al (2014) Clinical and biochemical effects of 810 nm diode laser as an adjunct to periodontal therapy: a randomized split-mouth clinical trial. Photomed Laser Surg 32(2):61–66
Nguyen NT, Byarlay MR, Reinhardt RA, Marx DB, Meinberg TA, Kaldahl WB (2015) Adjunctive non-surgical therapy of inflamed periodontal pockets during maintenance therapy using diode laser: a randomized clinical trial. J Periodontol 86(10):1133–1140
Yadwad KJ, Veena HR, Patil SR, Shivaprasad BM (2017) Diode laser therapy in the management of chronic periodontitis - a clinico-microbiological study. Interv Med Appl Sci 9(4):191–198
Birang R, Shahaboui M, Kiani S, Shadmehr E, Naghsh N (2015) Effect of nonsurgical periodontal treatment combined with diode laser or photodynamic therapy on chronic periodontitis: a randomized controlled split-mouth clinical trial. J Lasers Med Sci 6(3):112–119
Obradovic R, Kesic L, Mihailovic D, Jovanovic G, Antic S, Brkic Z (2012) Low-level lasers as an adjunct in periodontal therapy in patients with diabetes mellitus. Diabetes Technol Ther 14(9):799–803
Demirturk-Gocgun O, Baser U, Aykol-Sahin G, Dinccag N, Issever H, Yalcin F (2017) Role of low-level laser therapy as an adjunct to initial periodontal treatment in type 2 diabetic patients: a split-mouth, randomized, controlled clinical trial. Photomed Laser Surg 35(2):111–115
Al-Zahrani MS, Bamshmous SO, Alhassani AA, Al-Sherbini MM (2009) Short-term effects of photodynamic therapy on periodontal status and glycemic control of patients with diabetes. J Periodontol 80(10):1568–1573
Evangelista EE, Franca CM, Veni P et al (2015) Antimicrobial photodynamic therapy combined with periodontal treatment for metabolic control in patients with type 2 diabetes mellitus: study protocol for a randomized controlled trial. Trials. 16:229
Chandra S, Shashikumar P (2019) Diode laser - a novel therapeutic approach in the treatment of chronic periodontitis in type 2 diabetes mellitus patients: a prospective randomized controlled clinical trial. J Lasers Med Sci 10(1):56–63
Aoki A, Mizutani K, Schwarz F et al (2015) Periodontal and peri-implant wound healing following laser therapy. Periodontol 68(1):217–269
Holt SC, Kesavalu L, Walker S, Genco CA (1999) Virulence factors of Porphyromonas gingivalis. Periodontol 20:168–238
Kreisler M, Al Haj H, d’Hoedt B (2005) Clinical efficacy of semiconductor laser application as an adjunct to conventional scaling and root planing. Lasers Surg Med 37(5):350–355
Hakki SS, Bozkurt SB (2012) Effects of different setting of diode laser on the mRNA expression of growth factors and type I collagen of human gingival fibroblasts. Lasers Med Sci 27(2):325–331
Ishikawa I, Aoki A, Takasaki AA, Mizutani K, Sasaki KM, Izumi Y (2009) Application of lasers in periodontics: true innovation or myth? Periodontol 50:90–126
Sameera S, Aravind Kumar P, Nagasri M, Indeevar P, Raviraj K (2018) ENAP vs LANAP: assessment of revascularization using ultrasound Doppler flowmetry-a split-mouth randomized controlled clinical trial. Lasers Med Sci 33(6):1181–1188
Funding
The Research Project Coordination of Selcuk University (project no. 11202014) and The Scientific and Technological Research Council of Turkey (TUBITAK/SBAG-114S229) were supporters of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was reviewed and approved by the Ethics Commission of Selcuk University for human subjects (2013/57).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kocak, E., Sağlam, M., Arslan, U. et al. Effect of diode laser application as an adjunct to nonsurgical periodontal therapy on the reduction of red complex microorganisms in type 2 diabetics with chronic periodontitis. Lasers Med Sci 35, 1403–1410 (2020). https://doi.org/10.1007/s10103-020-02997-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-020-02997-1