Abstract
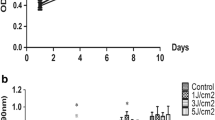
This study aimed to evaluate the effects of low-energy blue LED irradiation on the osteogenic differentiation of stem cells from the apical papilla (SCAPs). SCAPs were derived from human tooth root tips and were irradiated with 0 (control group), 1 J/cm2, 2 J/cm2, 3 J/cm2, or 4 J/cm2 blue light in osteogenic induction medium. Cell proliferation was analyzed using the 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay. Osteogenic differentiation activity was evaluated by monitoring alkaline phosphatase (ALP), alizarin red staining, and real-time polymerase chain reaction (RT-PCR). The results of the MTT assay indicated that SCAPs in the LED groups exhibited a lower proliferation rate than those in the control group, and there were statistically differences between the 2 J/cm2, 3 J/cm2, and 4 J/cm2 groups and the control group (P < 0.05). The results of the ALP and alizarin red analyses showed that blue LED promoted osteogenic differentiation of the SCAPs. And 4 J/cm2 blue light upregulates the expression levels of the osteogenic/dentinogenic genes ALP, dentin sialophosphoprotein (DSPP), dentin matrix protein-1 (DMP-1), and osteocalcin (OCN) in SCAPs. Our results confirmed that low-energy blue LED at 1 J/cm2, 2 J/cm2, 3 J/cm2, and 4 J/cm2 could inhibit the proliferation of SCAPs and promotes osteogenic differentiation of SCAPs. Further in vitro studies are required to explore the mechanisms of the effects by low-energy blue LED.




Similar content being viewed by others
References
Dixin C, Hongyu L, Mian W et al (2018) The origin and identification of mesenchymal stem cells in teeth: from odontogenic to non-odontogenic. Current Stem Cell Research & Therapy 13(1):39–45
Sonoyama W, Liu Y, Fang D et al (2006) Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One 1:e79
Nada OA, El BRM (2018) Stem cells from the apical papilla (SCAP) as a tool for endogenous tissue regeneration. Frontiers in Bioengineering and Biotechnology 6:103
Huang TJ, Sonoyama W, Liu Y et al (2008) The hidden treasure in apical papilla: the potential role in pulp/dentin regeneration and bioroot engineering. J Endod 34(6):645–651
Shiehzadeh V, Aghmasheh F, Shiehzadeh F et al (2014) Healing of large periapical lesions following delivery of dental stem cells with an injectable scaffold: new method and three case reports. Indian J Dent Res 25(2):248
Bakopoulou A, Leyhausen G, Volk J et al (2011) Comparative analysis of in vitro osteo/odontogenic differentiation potential of human dental pulp stem cells (DPSCs) and stem cells from the apical papilla (SCAP). Arch Oral Biol 56(7):709–721
Pereira MC, de Pinho CB, Medrado AR et al (2010) Influence of 670 nm low-level laser therapy on mast cells and vascular response of cutaneous injuries. J Photochem Photobiol B Biol 98(3):188–192
Basso FG, Oliveira CF, Kurachi C et al (2013) Biostimulatory effect of low-level laser therapy on keratinocytes in vitro. Lasers Med Sci 28(2):367–374
Pasternak-Mnich K, Ziemba B, Szwed A et al (2019) Effect of photobiomodulation therapy on the increase of viability and proliferation of human mesenchymal stem cells. Lasers Surg Med
Kim JE, Woo YJ, Sohn KM et al (2017) Wnt/β-catenin and ERK pathway activation: a possible mechanism of photobiomodulation therapy with light-emitting diodes that regulate the proliferation of human outer root sheath cells. Lasers Surg 49(10):940–947
Oliveira FA, Matos AA, Santesso MR et al (2016) Low intensity lasers differently induce primary human osteoblast proliferation and differentiation. J Photochem Photobiol B 163:14–21
Konig CJ, Buhner M, Murling G (2010) Blue-light irradiation regulates proliferation and differentiation in human skin cells. J Investig Dermatol 130(1):259
Arnolda G, Chien TD, Hayen A et al (2018) A comparison of the effectiveness of three LED phototherapy machines, single- and double-sided, for treating neonatal jaundice in a low resource setting. PLoS One 13(10):e0205432
Higuchi A, Shen PY, Zhao JK et al (2011) Osteoblast differentiation of amniotic fluid-derived stem cells irradiated with visible light. Tissue Eng A 17(21–22):2593–2602
Ginani F, Soares DM, Alexandre DORH et al (2018) Low-level laser irradiation induces in vitro proliferation of stem cells from human exfoliated deciduous teeth. Lasers Med Sci 33(1):95–102
Diao S, Lin X, Wang L et al (2017) Analysis of gene expression profiles between apical papilla tissues, stem cells from apical papilla and cell sheet to identify the key modulators in MSCs niche. Cell Prolif 50(3):e12337
Pagin MT, de Oliveira FA, Oliveira RC et al (2014) Laser and light-emitting diode effects on pre- osteoblast growth and differentiation. Lasers Med Sci 29(1):55–59
Zhu T, Wu Y, Zhou X et al (2019) Irradiation by blue light-emitting diode enhances osteogenic differentiation in gingival mesenchymal stem cells in vitro. Lasers Med Sci 34(7):1473–1481
Huang TJ, Gronthos S, Shi S (2009) Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res 88(9):792–806
Chueh LH, Huang TJ (2006) Immature teeth with periradicular periodontitis or abscess undergoing apexogenesis: a paradigm shift. J Endod 32(12):1205–1213
Estrela C, Alencar AH, Kitten GT et al (2011) Mesenchymal stem cells in the dental tissues: perspectives for tissue regeneration. Braz Dent J 22(2):91–98
Diogenes A, Hargreaves KM (2017) Microbial modulation of stem cells and future directions in regenerative endodontics. J Endod 43(9):S95–S101
Chrepa V, Pitcher B, Henry MA et al (2017) Survival of the apical papilla and its resident stem cells in a case of advanced pulpal necrosis and apical periodontitis. J Endod 43(4):561–567
Lin LM, Kim SG, Martin G et al (2018) Continued root maturation despite persistent apical periodontitis of immature permanent teeth after failed regenerative endodontic therapy. Australian Endodontic Journal the Journal of the Australian Society of Endodontology Inc 44(3):292–299
Huang GTJ, Yamaza T, Shea LD et al (2010) Stem/progenitor cell–mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng A 16(2):605–615
Na S, Zhang H, Huang F et al (2013) Regeneration of dental pulp/dentine complex with a three-dimensional and scaffold-free stem-cell sheet-derived pellet. J Tissue Eng Regen Med 10(3):261–270
Yan M, Wang L, Lei G et al (2013) Proliferation and osteo/odontoblastic differentiation of stem cells from dental apical papilla in mineralization-inducing medium containing additional KH2PO4. Cell Prolif 46(2):214–222
Marques, Márcia Martins, Diniz, Ivan a Márcia Alves et al (2016) Photobiomodulation of dental derived mesenchymal stem cells: a systematic review. Photomed Laser Surg 34(11):500–508
Lipovsky A, Nitzan Y, Gedanken A, Lubart R (2010) Visible light-induced killing of bacteria as a function of wavelength: implication for wound healing. Lasers Surg Med 42(6):467–472
Alghamdi KM, Kumar A, Moussa NA (2012) Low-level laser therapy: a useful technique for enhancing the proliferation of various cultured cells. Lasers Med Sci 27(1):237–249
Barboza CAG, Ginani F, Soares DM et al (2014) Low-level laser irradiation induces in vitro proliferation of mesenchymal stem cells. Einstein (Sao Paulo) 12(1):75–81
Soares D M , Ginani F , Henriques, ág uida Gomes et al (2015) Effects of laser therapy on the proliferation of human periodontal ligament stem cells. Lasers Med Sci 30(3):1171–1174
Mvula B, Mathope T, Moore T et al (2008) The effect of low level laser irradiation on adult human adipose derived stem cells. Lasers Med Sci 23(3):277–282
Zaccara IM, Ginani F, Mota-Filho HG et al (2015) Effect of low-level laser irradiation on proliferation and viability of human dental stem cells. Lasers Med Sci 30(9):2259–2264
Whelan HT, Smits RL, Buchmann EV et al (2001) Effect of NASA light-emitting diode irradiation on wound healing. J Clin Laser Med Surg 19(6):305–314
Posten W, Wrone DA, Dover JS et al (2005) Low-level laser therapy for wound healing: mechanism and efficacy. Dermatol Surg 31(3):334–340
Owen TA, Aronow M, Shalhoub V et al (1990) Progressive development of the rat osteoblast phenotype in vitro: reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrix. J Cell Physiol 143(3):420–430
Turrioni APS, Basso FG, Montoro LA et al (2014) Phototherapy up-regulates dentin matrix proteins expression and synthesis by stem cells from human-exfoliated deciduous teeth. J Dent 42(10):1292–1299
Li WT, Leu YC (2007) Effects of low level red-light irradiation on the proliferation of mesenchymal stem cells derived from rat bone marrow. Conf Proc IEEE Eng Med Biol Soc 2007:5830–5833
Soleimani M, Abbasnia E, Fathi M et al (2012) The effects of low-level laser irradiation on differentiation and proliferation of human bone marrow mesenchymal stem cells into neurons and osteoblasts—an in vitro study. Lasers Med Sci 27(2):423–430
Ballini A, Mastrangelo F, Gastaldi G et al (2015) Osteogenic differentiation and gene expression of dental pulp stem cells under low-level laser irradiation: a good promise for tissue engineering. J Biol Regul Homeost Agents 29(4):813–822
Borzabadi-Farahani A (2016) Effect of low-level laser irradiation on proliferation of human dental mesenchymal stem cells; a systemic review. J Photochem Photobiol B Biol 162:577–582
Li R, Peng L, Ren L et al (2009) Hepatocyte growth factor exerts promoting functions on murine dental papilla cells. J Endod 35(3):382–388
Feng JQ, Huang H, Lu Y et al (2003) The dentin matrix protein 1 (Dmp1) is specifically expressed in mineralized, but not soft, tissues during development. J Dent Res 82(10):776–780
Wang S, Mu J, Fan Z et al (2012) Insulin-like growth factor 1 can promote the osteogenic differentiation and osteogenesis of stem cells from apical papilla. Stem Cell Res 8(3):346–356
Funding
This work was supported by the Luzhou Municipal People’s Government-Southwest Medical University science and technology strategic cooperation projects of China (no. 2017LZXNYD-T03), Luzhou Municipal Science and Technology Bureau of China (no. 2016-R-70(13/24)). The reagents of this study were supported by these funds that all came from Southwest Medical University.
Author information
Authors and Affiliations
Contributions
Yaoyao Yang and Tingting Zhu designed the research, conducted the experiments, and wrote the paper. Yan Wu and Chunxia Shu conducted the experiments. Qiang Chen, Juan Yang, and Xiang Luo performed the data analyses and edited the manuscript. Yao Wang designed the research, supervised the study, and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the study were in accordance with the Ethics Committee of the Affiliated Hospital of Stomatology Southwest Medical University Certificate (contract grant 20180314001) and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, Y., Zhu, T., Wu, Y. et al. Irradiation with blue light-emitting diode enhances osteogenic differentiation of stem cells from the apical papilla. Lasers Med Sci 35, 1981–1988 (2020). https://doi.org/10.1007/s10103-020-02995-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-020-02995-3