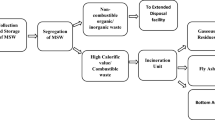
Abstract
The efforts to encourage sustainable development have brought to attention the use of secondary materials in civil engineering applications. Global experimental data suggest that municipal solid waste (MSW) incineration bottom ash (IBA) can potentially replace natural aggregates in field applications. However, IBA is currently disposed in open dumps or sanitary landfills in India. Assessing contaminant leaching from IBA is crucial to ascertain its options for disposal or reuse. The current study analyzes the mineralogy, chemical constituents, organic content, and leaching of contaminants from IBA collected from two incineration plants in Delhi. The study takes into consideration the effect of particle size, liquid-to-solid (L/S) ratio, and pH on contaminant leaching. The experimental results revealed that the leaching concentration of contaminants and organic content of IBA decreases with an increase in particle size. A considerable influence of pH was noted on the leaching of all contaminants except chlorides, sulfates, and antimony. The results further indicate that IBA is suitable for disposal in non-hazardous waste landfills regardless of particle size at its intrinsic pH. However, it is recommended to dispose IBA in monofils as co-disposal with MSW in sanitary landfills can cause the pH of IBA to shift toward the acidic range, which may exacerbate the contaminant leaching from IBA. The comparison of leaching test results with international regulatory standards for reuse demonstrates that using IBA in unbound applications is feasible in paved roads and earthworks subjected to minimal infiltration of water.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The fundamental solid waste management principle emphasizes maximum material utilization with minimal disposal in landfills (Wagner and Raymond 2015; Van Fan et al. 2021). Several studies (Bureau EI 2005; Ng et al. 2014; Tan et al. 2015) have indicated incineration to be an effective waste management strategy for achieving a mass reduction of 75–80% and also generating electricity and heat (renewable energy). In India, incineration is employed to handle enormous quantities of municipal solid waste (MSW) in metropolitan cities such as Delhi to minimize the waste reaching sanitary landfills (CPCB 2021; Tripathy 2018). Typically, in MSW incineration facility, the furnace temperature ranges from 850 to 1000oC, and the process yield residues comprising primarily of bottom ash (80–90%) and minor quantities of fly ash (10–20%) (DEFRA 2013; Tang et al. 2015). While fly ash has mainly been categorized as hazardous in several countries, bottom ash can potentially be recycled in civil engineering applications (Chang and Wey 2006; Dou et al. 2017; Lam et al. 2010; Tang et al. 2015). However, in India, these residues are currently dumped in open dumps, MSW landfills, or mining pits due to the dearth of scientific studies and the absence of any national regulatory framework governing their reuse (Gupta et al. 2021a). Mining of sand and gravel has been regulated in India due to escalating environmental concerns (MoEF&CC 2020). Reusing incineration bottom ash (IBA) as a substitute for natural aggregates would provide an alternative to conventional aggregates and also reinforce the sustainable development goals (SDGs 2018) of the country.
Ascertaining the options for disposal or reuse of secondary materials, such as IBA, necessitates understanding its impact on the surrounding environment, which is chiefly studied using leaching test methods. Several test methods have been established to comprehend the leaching of contaminants from any material (Blasenbauer et al. 2020; ISWA 2013; Kosson et al. 2014). Some of the commonly used test methods include batch leaching using de-ionized (DI) water (BS EN 12457-2/4 2002), batch leaching based on acidic leachant (USEPA 2011), pH-dependent batch leaching (BS EN 14429 2015; USEPA 1313 2013) and column leaching (BS EN 14405 2017; USEPA 1314 2013). Batch leaching, the static leaching method, is simple and quick, which estimate contaminant leaching from a material at chemical equilibrium condition achieved using a fixed liquid-to-solid ratio (L/S), contact duration, and pH. However, batch leaching fails to capture the leaching characteristics in dynamic conditions (Grathwohl and Susset 2009). Column leaching is comparatively more complicated than batch leaching, which essentially captures the release of contaminants from the material as a function of the L/S ratio and more closely reflects the leaching process under field conditions (Grathwohl and Susset 2009; Kalbe et al. 2008; Yin et al. 2018). pH-dependent batch leaching tests are curated to examine the fluctuations in contaminant leaching from a material as a function of pH which is regarded as crucial in understanding the impact of varied environmental conditions (van der Sloot 1996; Dijkstra et al. 2006; Ai et al. 2019). None of the leaching methods alone is sufficient enough to decipher the intricate process of contaminant leaching from IBA. Leaching test results from the three methods, i.e., batch leaching, column leaching, and pH-dependent batch leaching, when studied in conjunction with one another, can combinedly contribute toward a better understanding of the leaching process from the material (Grathwohl and Susset 2009; Quina et al. 2011; Kosson et al. 2014).
Several studies (see Table S1 in supplementary material) have conducted various leaching tests to ascertain the disposal and reuse options of IBA. It is evident from Table S1 that the leaching tests have been performed on varied particle size ranges of IBA, and only a few studies have examined the influence of particle size on contaminant leaching (Arickx et al. 2006; Huber et al. 2019; Loginova et al. 2019; Sormunen and Rantsi 2015). Leaching studies based on acidic leachant have mostly been conducted in Asian countries (Chang and Wey 2006; Nikravan et al. 2020; Song et al. 2004; Xuan and Poon 2018) and not from the European nations. It can also be noted that most of the studies have estimated contaminant leaching at the intrinsic pH of IBA, i.e., using DI water (Blanc et al. 2018; del Valle-Zermeno et al. 2013; Ginés et al. 2009; Lidelow and Lagerkvist 2007; Saikia et al. 2015). Some researchers have exclusively investigated the role of pH (Dijkstra et al. 2006; Meima and Comans 1999; Zhang et al. 2008) on IBA. Furthermore, the influence of the L/S ratio on contaminant leaching as determined by column leaching tests is less studied than batch leaching (Di Gianfillipo et al. 2018; Izquierdo et al. 2008; Sormunen and Rantsi 2015). Arickx et al. (2006) and Hjelmar et al. (2007) studied leaching from IBA at its intrinsic pH as well as tested the influence of pH and L/S ratio. However, Hjelmar et al. (2007) have taken into consideration only a few contaminants, i.e., chloride, sulfates, nickel, and antimony. No comprehensive study exists to the authors' best knowledge that has addressed the influence of particle size, L/S ratio, and pH on the leaching of contaminants from IBA. This is the first in-depth and comprehensive research study being reported from a country where MSW incineration is relatively new (with only 8 operational incineration plants) and is being scaled up significantly with more than 50 incineration plants in construction (CPCB 2021). This study also addresses the behavior of individual contaminants under varying conditions, and a comparison has been made with available literature for ready reference to other researchers.
Several studies (Chang et al. 2004; Tang et al. 2015; Vaitkus et al. 2018; Gupta et al. 2021a, 2021b) have demonstrated variable characteristics of IBA due to differences in MSW composition, pre-processing of waste, and operating conditions of the incinerator. Gupta et al. (2021b) have studied the leaching characteristics of IBA from Indian MSW incineration plants, but the study is limited to IBA fraction passing 4.75 mm and at intrinsic pH conditions.
The present work characterizes IBA from two MSW incineration plants in Delhi for mineralogy, chemical constituents, and organic content and investigates in detail the leaching of contaminants from the same. The study takes into consideration the effect of particle size, liquid-to-solid (L/S) ratio, and pH on contaminant leaching. Conventionally used TCLP (toxicity characteristics leaching procedure) (USEPA 2011) leaching test was not used in the present study as Intrakamhaeng et al. (2019) have demonstrated it to be unsuitable for alkaline residues such as IBA. The suitability of IBA for disposal or reuse was adjudged by gauging the leaching test results with international regulatory standards. The study provides valuable insight into the leaching behavior of IBA and recommends options for disposal or reuse. The study would be beneficial for various stakeholders and policymakers to determine the fate of IBA.
Material and methodology
Material collection
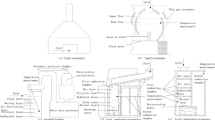
Presently, Delhi has three MSW incineration facilities that operate using moving-grate technology. Approximately four to five tons of IBA was collected from two of these facilities. The samples were air-dried for approximately a week under a shed and then screened to obtain three different sized IBA fractions (Fig. S1 in supplementary material): (a) overall material (OM) passing 31.5 mm, which accounted for more than 90% of the total material; (b) soil-like material (SLM) passing through 4.75 mm sieve; (c) gravel-sized material (GSM) with particles in size range between 4.75 and 31.5 mm. The representative samples from each IBA fraction were obtained using coning and quartering methods for further testing.
Experimental investigation
The experimental investigation conducted on the IBA samples is illustrated in Table 1 and explained subsequently.
Chemical characterization
X-ray diffraction (XRD) technique was used to determine the minerals present in IBA samples. For this purpose, the samples were pulverized using a ball mill to pass a 0.075 mm sieve and oven dried at 105 ± 5 °C. XRD analysis was conducted using Rigaku miniflex 600 with Cu-Kα radiation for the detection angle ranging between 10° and 80° with a scanning rate of 4°/min and a step width of 0.02°.
X-ray fluorescence (XRF) was used for the quantitative analysis of major and minor elements in IBA using Xenemetrix EX-6600SDD. The sample preparation for XRF analysis involved igniting the oven-dried pulverized IBA samples (passing 0.075 mm) at 950 ± 25 °C in a muffle furnace (Rendek et al. 2007; Santos et al. 2013), placing the same in plastic cups and covering them with prolene film (Brouwer 2006; Malik et al. 2016).
Aqua regia acid digestion (USEPA 3050 1986) and inductively coupled plasma mass spectrometry (ICP-MS) were used to obtain the total concentration (environmentally available) of the trace metals. For this purpose, approximately 0.2 g of the pulverized sample passing 0.075 mm was mixed with 4.5 mL of hydrochloric acid and 1.5 mL of nitric acid, and then microwave-digested (Chimenos et al. 1999; Gupta et al. 2021b; Holm and Simon 2017; Li et al. 2019). The trace metal(loid) concentration was determined on the filtered extract using ICP-MS. The total content of sulfates and chlorides was determined by BS: 1377-3 (2018) standard. The excess HCl extraction method was used for determining sulfates, whereas chlorides were determined by titrating with silver nitrate and thiocyanate solutions.
The organic content of IBA was determined using loss on ignition (LOI) tests. The samples were oven dried at 105 ± 5 °C, and about 100 g of representative samples were then ignited in a muffle furnace at 550 ± 10 °C for 4 h to determine the LOI of IBA (Arm 2004; Gupta et al. 2021b; Yao et al. 2012).
Leaching tests
DI water batch leaching tests were conducted as per the standard BS EN 12457-2/4 (2002). The IBA samples were crushed to pass a 10-mm sieve and about 90 g sample was mixed with 900 mL of DI water using end-to-end rotator for 24 h. Thereafter, the leachate samples were filtered through a 0.45 μm filter. The filtered extract was analyzed for pH, chlorides, and sulfates immediately and for trace metal(loid)s testing stored at a pH of less than 2 and temperature of 4 °C. The samples were analyzed for trace metal(loid)s by ICP-MS. Sulfates and chlorides were determined using the turbidimetric method and titration using the silver-nitrate solution, respectively (APHA et al. 2012).
DI water column leaching tests (BS EN 14405 2017) were performed to determine the influence of the L/S ratio on contaminant leaching. The IBA samples were crushed to pass a 10-mm sieve. An acrylic column of height 32 cm and diameter 10 cm was filled with crushed IBA in five layers for a total of 30 cm. The column was packed with a centimeter-thick layer of clean sand and filter paper on either side. The column was first saturated with up-flowing DI water with a flow rate of 0.9 ± 0.1 mL/min. After saturation, the column was left undisturbed for an equilibration period of three days. Thereafter, the column leaching test was commenced using the same flow rate as used for achieving saturation. Leachate samples were collected at the seven L/S ratios (in L/kg), i.e., 0.1, 0.2, 0.5, 1.0, 2.0, 5.0, and 10.0 and filtered through a 0.45 μm filter. The filtrate was analyzed for pH, sulfates, chlorides, and trace metal(loid)s. The samples were acidified at pH below 2 and stored at a temperature of 4 °C for trace metal(loid)s before being analyzed by ICP-MS.
Static pH-dependent leaching tests were conducted on IBA samples using BS EN 14429 (2015). The tests were performed at pH ranging from 4 to 12 (4, 5, 6, 7, 8, 9, 10, and 11.5). IBA was mixed with the leachant at a liquid-to-solid (L/S) ratio of 10 L/kg. The leachant consisted of a combination of nitric acid (0 to 5 mL) and de-ionized water. The amount of nitric acid added to maintain the required pH was determined from pre-titration experiments. The suspension obtained was filtered through a 0.45 μm filter, and the eluate was analyzed for pH, sulfates, chlorides, and heavy metal(loid)s. Similar to DBL and DCL, eluates obtained were stored at a pH of less than 2 and a temperature of 4 °C for trace metal(loid)s testing.
The leachability of the contaminants was calculated from the two leaching test methods, i.e., DBL and DCL, as the ratio of the leaching concentration to the total content and expressed as percentage. The leachability of contaminants from the PBL method was calculated similarly to DBL and DCL, except that the maximum leaching concentration obtained for the studied pH range was used in the calculation.
Gupta et al. (2021b) identified ten contaminants of concern, namely Cd, Cr, Cu, Mo, Ni, Pb, Sb, Zn, Cl− and \({\text{SO}}_{4}^{2-}\), which should mandatorily be investigated for IBA. Hence, the present work is restricted to testing and discussion on these contaminants.
Assessment of options for disposal and reuse
In India, solid waste management rules (SWM rules 2016) stipulate TCLP tests for ascertaining the disposal of incineration ash. However, a recent study (Intrakamhaeng et al. 2019) demonstrated that the TCLP test is unsuitable for alkaline residues such as IBA. Furthermore, India does not have any prescriptive regulation for assessing the reuse options of IBA. European countries, on the contrary, have well-established regulatory specifications for determining the fate of incineration ash. Some of these regulations, as depicted in Table 2, have been employed in the present work for adjudging the results of leaching tests. The threshold limits specified for the contaminants for disposal and reuse of IBA are shown in Table S2. Additional details on reuse regulations are provided in Table S3.
It should be noted that the basis for the assessment of the fate of IBA in the present study is restricted to the leaching of selected inorganic contaminants. The study of organic contaminants is beyond the scope of the present work.
Results and discussion
Chemical characteristics of IBA
The minerals identified in the three IBA fractions are shown in Fig. 1. The minerals were identified by analyzing peak intensities obtained in XRD results with the International Centre for Diffraction Data database and comparing them with the literature on IBA (Keppert et al. 2012; Lin et al. 2015; Margallo et al. 2014; Polettini et al. 2005; Tang et al. 2015). Since the samples from the two incineration plants exhibited similar XRD patterns, only the typical response is presented in Fig. 1. The three size fractions of IBA are observed to constitute similar minerals but have varying peak intensities. Quartz is the predominant mineral followed by the presence of calcite, anhydrite, feldspar, magnetite, and halite. SLM possesses high calcite and anhydrite peak intensities than GSM and OM (Lin et al. 2015; Tang et al. 2015). In contrast, magnetite peak intensity increases as the particle size increases (Lin et al. 2015; Tang et al. 2015). According to Tang et al. (2015), this could be attributed to the difference in hardness between crystalline phases; for instance, calcite and anhydrite minerals are softer and may crush and hence, present in higher quantities in SLM.
The other chemical characteristics of IBA are presented in Table 3. SiO2, CaO, Al2O3, and Fe2O3 are the major chemical constituents present in the three size fractions of IBA which accounts for nearly 80–85% of the total. The higher content of Si, Ca, Al, and Fe in IBA is also borne out from the results of XRD analysis which reveals quartz, calcite, and feldspars to be the predominant minerals. The minor chemical constituents of IBA include MgO, K2O, Na2O, SO3, P2O5, Cl, and TiO2. Indian IBA is observed to have higher silica content in comparison with the reported literature from other nations (Chang and Wey 2006; Keppert et al. 2012; Polettini et al. 2005; Rendek et al. 2007; Shen et al. 2021). This might be due to higher quantities of street sweepings and mixing of drain silt or construction and demolition waste with MSW in India (Kaza et al. 2018; Priti and Mandal 2019). It should also be noted that the SiO2 content of GSM is higher, while CaO, Cl, and SO3 content is lower in comparison with SLM and OM fractions. Alam et al. (2019) also reported that the finer fraction of IBA has lower SiO2 and higher CaO and Cl in comparison with coarser fractions.
The total content of Ba, Cr, Cu, and Zn was found to be above 200 mg/kg followed by Ni and Pb having concentrations in the range of 50–100 mg/kg, and the remaining trace metal(loid)s have concentrations below 25 mg/kg. The total content of sulfate and chloride is observed to vary in the range of 0.23–1.07% and 0.52–1.83%, respectively. The total content of most of the trace metal(loid)s as well as chlorides and sulfates is observed to be lowest for GSM, followed by OM, and highest for SLM. This illustrates that most of the trace metal(loid)s, chlorides, and sulfates are concentrated in finer fractions of IBA which is in agreement with the previous studies (Loginova et al. 2019; Sormunen and Rantsi 2015).
The organic content (or LOI 550 °C) follows the sequence: SLM (3.5–4.6%) > OM (2.5–2.9%) > GSM (1.4–1.6%). The results are similar to the values reported in the literature (Le et al. 2018; Lidelow and Lagerkvist 2007; Tang et al. 2015; Traina et al. 2007). Several countries have restricted the LOI content for reuse in field applications, such as 3% by French regulation (Government of French Republic 2011) and 5% by Dutch regulation (Soil Quality Decree 2007). Indian IBA meets the abovementioned LOI limits, except for SLM which exceeds the French limits.
Leaching characteristics of IBA
The results of the three categories of leaching tests, i.e., DBL, DCL, and PBL, are shown in Figs. 2, 3, and 4, respectively. The leachability of contaminants as calculated from the test results is presented in Table 4. The intrinsic pH of IBA, as obtained from DBL and DCL leaching test method, varies between 10.5 and 11.5 which is similar to the values reported in the previous studies (Caviglia et al. 2019; Hyks et al. 2011; Lidelöw and Lagerkvist 2007; Minane et al. 2017; Nikravan et al. 2020). The results are discussed in detail in the subsequent sections with respect to each contaminant.
Cadmium
The DBL test (Fig. 2) results reveal Cd to be below the detection limit in GSM fraction while the values are 30–33% lower in OM than in SLM. The DCL test results show steady depletion of Cd until the L/S ratio of 1 L/kg, which is followed by negligible dissolution with a further increase in L/S. A similar pattern is observable in the previous studies (Di Gianfillipo et al. 2018; Izquierdo et al. 2008). In PBL tests (Fig. 4), the leaching of Cd is significantly affected by a change in pH (Di Gianfillipo et al. 2018). The tests suggest that Cd is mainly released under acidic conditions with leaching increasing by 2–3 magnitude orders in comparison with alkaline conditions. A similar trend was observed by Quina et al. (2009). The leaching of Cd reduces significantly at moderate to highly alkaline conditions (pH of 6–12) which is possibly due to its adsorption by hydrous ferric oxide (Dijkstra et al. 2006; Zhang et al. 2008) or due to the presence of insoluble otavite (CdCO3) (Ai et al. 2019; Meima and Comans 1999). The leachability of Cd in DBL and DCL tests is only 0.2% (Table 4), suggesting that its leaching is not a matter of concern for the intrinsic pH conditions. However, the leachability is much higher (~ 70%) for acidic conditions.
Chromium
The DBL test results (Fig. 2) reveal leaching of Cr from the GSM is much lower in comparison with OM and SLM. Sormunen and Rantsi (2015) also studied the effect of particle size of IBA and observed similar behavior with average leaching concentration varying from 0.4–0.7 mg/kg for GSM to 1.6–3 mg/kg for SLM. DCL tests (Fig. 3) indicate the inability of Cr to leach at very low L/S (till L/S 0.2 L/kg) which is followed by rapid dissolution until L/S 2 L/kg and insignificant dissolution with further increase in L/S (Gori et al. 2011; Izquierdo et al. 2008; Olsson et al. 2009). PBL test results (Fig. 4) show that the leaching of Cr is highest in acidic conditions which decreases with increase in pH until neutral pH, and thereafter, remains almost constant. Quina et al. (2009) associated low leaching of Cr at highly alkaline pH with the absorption of Cr by ettringite. Zhang et al. (2008) indicated that chromium oxide (Cr2O3) is responsible for controlling the leaching of Cr in alkaline conditions. The leachability of Cr from DBL and DCL tests is 0.08–0.14% (Table 4) which does not change significantly in PBL (0.98–1.05%), suggesting that most of the chromium remains bound in the solid matrix and is unavailable for leaching.
Copper
The DBL test results (Fig. 2) demonstrate greater leaching of Cu from SLM, followed by OM and least from GSM. DCL test results (Fig. 3) indicate that Cu exhibits rapid dissolution till L/S 1L/kg, and thereafter, the increase in dissolution becomes insignificant with the increase in L/S (Di Gianfillipo et al. 2018; Gori et al. 2011: Izquierdo et al. 2008). pH-dependent tests (Fig. 4) indicate Cu leaching decreases with increase in pH. Dijkstra et al. (2006) suggested malachite to control the leaching of Cu for pH above 8. Whereas, Zhang et al. (2008) attributed copper oxide to govern the leaching of Cu for pH above 8 and malachite in the pH range 5–7. However, several studies (Dijkstra et al. 2006; Hyks and Astrup 2009; Olsson et al. 2009; Santos et al. 2013) have regarded the availability of dissolved organic carbon (DOC) to significantly influence the leaching of Cu as DOC forms organic complexes with Cu which accentuates the mobility of Cu. The leachability of Cu at intrinsic pH, as obtained from DBL and DCL testing, is low (∼ 1%), which increases up to 9–10% (Table 4) under acidic conditions.
Molybdenum
The DBL test results (Fig. 2) indicate Mo leaching from coarser IBA fraction, i.e., GSM, to be approximately 10 times lower in comparison with finer IBA fraction, i.e., SLM. Leaching of Mo from OM is approximately 30–40% of SLM. The DCL test results (Fig. 3) demonstrate leaching of Mo to be solubility controlled as it increases with an increase in L/S and shows progressive depletion (Dou et al. 2017; Di Gianfilippo et al. 2018; Izquierdo et al. 2008). The PBL test results (Fig. 4) reveal leaching of Mo to be maximum at neutral to slightly alkaline pH (pH of 7–8) which diminishes under acidic and alkaline pH. Previous studies have observed a similar leaching pattern of Mo due to changes in pH (Ai et al. 2019; Santos et al. 2013; Van Gerven et al. 2005). Leaching of Mo is associated with iron molybdate or wulfenite under acidic conditions (pH < 6) (Dijkstra et al. 2006; Meima and Comans 1999). Whereas, the formation of precipitates of calcium molybdate and oxyanion substitution of sulfate in ettringite is regarded responsible for reduced solubility of Mo under alkaline conditions (Van Gerven et al. 2005). The leachability of Mo is between 18 and 25% at the intrinsic pH of IBA which increases to 55–60% in the pH range 7–8 (Table 4).
Nickel
DBL test results (Fig. 2) demonstrate that the leaching of Ni from OM is 15–25% lower than that of SLM, whereas leaching from GSM is insignificant in comparison with SLM or OM. As evident from DCL test results (Fig. 3), leaching of Ni is availability controlled as it exhibits rapid depletion in the initial L/S ratios (until L/S 1 L/kg), and thereafter, the increase in dissolution becomes insignificant with the increase in L/S (Di Gianfillipo et al. 2018; Izquierdo et al. 2008). The PBL tests (Fig. 4) indicate that in acidic pH conditions, Ni has higher leaching concentrations, which reduces with an increase in pH. This is also evident from the fact that the leachability (17–25%) of Ni in acidic conditions decreases to 0.4–1.4% at intrinsic pH (Table 4). At pH above 7, nickel hydroxide governs the leaching of Ni as it is insoluble and neutralizes the pH, thereby reducing the leaching of Ni (Dijkstra et al. 2006; Zhang et al. 2008). Moreover, hydrous ferric oxide (HFO as FehONi+) can also affect the leaching of Ni in the pH range of 6–9. It can adsorb Ni ions or form a complex with Ni, thereby reducing its leaching concentration (Zhang et al. 2008; Santos et al. 2013).
Lead
The DBL test results (Fig. 2) reveal Pb to be below the detection limit in the GSM fraction, while the values are 60–70% higher in SLM than in OM. The DCL test results (Fig. 3) indicate the inability of Pb to leach at low L/S (0.1–0.2 L/kg), followed by rapid dissolution till L/S 2 L/kg, and insignificant increase in dissolution with further increase in L/S (Di Gianfillipo et al. 2018; Gori et al. 2011; Lynn et al. 2016). In PBL tests (Fig. 4), Pb shows amphoteric nature, with the lowest leaching at around neutral pH and concentration increasing on both sides. The variation in pH significantly influences the leaching of Pb, which is evident from 3 magnitude orders of difference between the minimum (0.003–0.007 mg/kg) and maximum (5.1–7.2 mg/kg) leaching concentrations. The acidic environment enhances the solubility of Pb, hence, increasing its leaching. At neutral pH conditions, the formation of compounds with complexing agents such as DOC (Dijkstra et al. 2006) or adsorption of Pb on iron oxide surfaces (Oehmig et al. 2015) results in the leaching suppression of Pb. In alkaline conditions, Pb leaches from IBA; however, the leaching is less than that in acidic conditions due to the formation of lead hydroxide precipitates (Zhang et al. 2008; Gori et al. 2011). The leachability of Pb (Table 4) in DBL and DCL tests is insignificant (0.05–0.08%), and it increases to 5–7% in acidic environments. Thus, indicating that most of Pb is unavailable for leaching and remains bound in the solid matrix.
Antimony
The DBL test results (Fig. 2) indicate higher leaching of Sb from SLM, followed by OM, and the least from GSM. DCL test results (Fig. 3) reveal leaching of Sb to be solubility controlled as it increases steadily with increasing L/S (Di Gianfilippo et al. 2018; Dou et al. 2017; Yin et al. 2018) and exhibits progressive and slow dissipation of concentration (Bruder-Hubscher et al. 2001; Izquierdo et al. 2008). In PBL tests (Fig. 4), the leaching of Sb resembles Mo, with maximum leaching observed in the pH range of 7–8 and then decreasing in alkaline and acidic conditions. Out of observed metal(loid)s, Sb is least affected by the change in pH, evident from the minimal difference in maximum (0.73–1.27 mg/kg) and minimum (0.08–0.24 mg/kg) leaching concentrations. Sb leaching is governed by the adsorption of Sb on ferrous and aluminum oxide surfaces (Ai et al. 2019; Ginés et al. 2009). At higher pH, the leaching of Sb decreases because of the formation of calcium antimonate precipitates (Cornelis et al. 2006; Dijkstra et al. 2006). The transformation of ettringite to gypsum at pH below 8 increases the solubility of Sb, thereby increasing the leaching (Ai et al. 2019; Cornelis et al. 2006; Santos et al. 2013). The leachability of Sb increases from 5 to 8% at intrinsic pH (in DBL and DCL tests) to around 26% at pH of 7–8 (in PBL tests), as shown in Table 4.
Zinc
The DBL test results (Fig. 2) indicate that the leaching of Zn in the GSM fraction is two magnitude orders lower than that in SLM, and the leaching from the OM fraction is comparable to SLM. DCL test results (Fig. 3) show rapid depletion in Zn as indicated by the steep initial slope of the curve, until L/S 1 L/kg, which flattens out with the further increase in L/S (Izquierdo et al. 2008; Yin et al. 2018). The PBL tests (Fig. 4) illustrate that the leaching of Zn is also amphoteric, with minimum leaching concentrations at a pH of 7–8. Akin to Pb, Zn leaching varies significantly with pH, as indicated by the difference in maximum (63.2–85.8 mg/kg) and minimum (0.01–0.03 mg/kg) leaching concentrations. The presence of willemite (Zn2SiO4) affects the leaching of Zn, as it enhances solubility in acidic conditions and reduces solubility in alkaline conditions (Dijkstra et al. 2006; Meima and Comans 1999; Zhang et al. 2008). In the pH range of 5–8, the formation of precipitates of zinc carbonate suppresses the leaching of Zn (Zhang et al. 2008). The leachability of Zn (Table 4) is low in DBL and DCL tests (0.04–0.06%) and increases to around 11% in PBL tests in acidic conditions.
Chlorides and sulfates
The DBL test results (Fig. 2) indicate that all three fractions of IBA have noticeable chlorides and sulfates leaching concentrations. In the GSM fraction, chlorides and sulfates leaching is approximately half of SLM, whereas, in OM, leaching is 75–80% less than that of SLM. The DCL test results (Fig. 3) illustrate the leaching of Cl− to be availability controlled as indicated by the steep initial slope of the curve, until L/S 1 L/kg, which flattens out with the further increase in L/S (Åberg et al. 2006; Bruder-Hubscher et al. 2001; Di Gianfilippo et al. 2018; Izquierdo et al. 2008; Lidelow and Lagerkvsit 2007). On the contrary, sulfate leaching is solubility controlled and exhibits progressive depletion with the increase in L/S (Di Gianfilippo et al. 2018; Dou et al. 2017; Izquierdo et al. 2008). The pH-dependent tests (Fig. 4) indicate that Cl− and \({\text{SO}}_{4}^{2-} \mathrm{}\) leaching is less affected by pH variations in comparison with trace metal(loid)s. This is evident from the minimal difference between the maximum and minimum leaching concentrations. Meima and Comans (1999) and Quina et al. (2009) also observed that the leaching of Cl− does not vary significantly with a change in pH. The leaching concentration of sulfates increases with a reduction in pH from alkaline to neutral conditions and thereafter remains almost constant (Dijkstra et al. 2006; Meima and Comans 1999; Quina et al. 2009). At pH above 9, ettringite controls the solubility of \({\text{SO}}_{4}^{2-}\), whereas, at pH below 9, gypsum governs the leaching of \({\text{SO}}_{4}^{2-}\)(Dijkstra et al. 2006; Meima and Comans 1999). The leachability varies from 30 to 45% for Cl− and 25 to 42% for \({\text{SO}}_{4}^{2-}\) from DBL/DCL to PBL tests, as indicated in Table 4.
Assessment of leaching characteristics of IBA
With disposal criteria
The comparison of DBL (Fig. 2) test results with European Union disposal limits prescribed for BS EN 12457–2/4 test (EU Council 2003) reveals the concentration of Cr, Cu, Mo, Sb, Cl− and \({\text{SO}}_{4}^{2-}\) in SLM, Cl− and \({\text{SO}}_{4}^{2-}\) in GSM, and Cu, Sb, Cl− and \({\text{SO}}_{4}^{2-}\) to exceed the EU-IW limits. However, contaminant leaching from neither of the three fractions, i.e., SLM, GSM, and OM supersede the EU-NHW limits. Therefore, DBL test results demonstrate IBA to be non-inert but non-hazardous regardless of the particle size at its intrinsic pH indicating that it can safely be disposed in non-hazardous waste landfills.
DCL test results (at L/S 0.1 L/kg) are compared with the European Union disposal limits prescribed for BS EN 14405 (2017) test method in Table S4. As evident from Table S4, the leaching of most of the contaminants, i.e., Cu, Ni, Sb, Cl−, and \({\text{SO}}_{4}^{2-}\), exceed the EU-IW disposal limits. However, no contaminant surpasses the EU-NHW limits. Therefore, DCL test results demonstrate OM fraction of IBA is unsuitable for disposal in inert waste landfills but safe for disposal in non-hazardous waste landfills.
The PBL test results are compared with European Union disposal limits prescribed for EN 12457-2/4 (2002) test method, as suggested by Quina et al. (2009). The comparison with EU-IW disposal limits illustrates that IBA is unsuitable for disposal to inert waste landfills as leaching of most of the inorganic constituents consistently exceed the EU-IW limits, irrespective of the pH condition. The comparison of results with EU-NHW limits demonstrates that IBA is suitable for disposal in non-hazardous waste landfills when the pH of IBA lies above 10. However, the increase in leaching of Sb in the pH range 7 to 10, while that of Cd and Zn in the pH range 4–5 is concerning and significant enough to cause IBA to be categorized as hazardous. This suggests that disposal of IBA in non-hazardous waste landfills is favorable for fresh IBA with an intrinsic pH over 10. However, weathering of IBA with the passage of time can cause pH to fall in the range of 8 to 10 (Arickx et al. 2006; Loginova et al. 2019; Van Gerven et al. 2005). This can be a cause of concern, as increased Sb leaching may not be conducive to its disposal in non-hazardous waste landfills.
Several studies (Caviglia et al. 2019; del Valle-Zermeño et al. 2013; Ginés et al. 2009; Hyks et al. 2011; Lidelöw and Lagerkvist 2007; Minane et al. 2017; Nikravan et al. 2020) have depicted IBA as non-hazardous. Only limited data is available (Arickx et al. 2006; Sormunen and Rantsi 2015; Tang et al. 2016) that has emphasized the hazardous nature of IBA due to exceedance in the leaching of Sb. The insufficient data on the hazardous nature of IBA can be attributed to the fact that most of the studies on IBA have been conducted on fresh samples, wherein Sb mostly meets the EU-NHW criteria.
PBL test results further emphasize that it is crucial to ensure that the pH of IBA does not shift toward the acidic range while disposing the same. The shift in pH of IBA toward the acidic range is a possibility when co-disposing IBA with MSW in sanitary landfills as the decomposition of MSW leads to the generation of acetic acid, fulvic acid, and other organic acids (Ahmed and Lan 2012; Kulikowska and Klimiuk 2008). Therefore, it is recommended to dispose IBA in monofils than co-disposing the same with MSW in sanitary landfills as the latter option may aggravate the leaching of contaminants from IBA.
With reuse criteria
The comparison of DBL test results with reuse limits (Fig. 2) illustrates that OM, SLM, and GSM can possibly be reused in roads as per the Belgium criteria (B1), in backfills and paved roads as per the French (Fr1) or Finland (F1) criteria. Utilization of IBA in covered embankments is not feasible as the concentration of sulfates and chlorides exceed the Finland criteria (F2) for covered embankments.
The comparison of the DCL test results with reuse criteria (Fig. 4) suggests the feasibility of reuse of OM in roads as per the Belgium criteria (B2), in paved roads as per the Finland criteria (F1), or isolated reuse as per the Dutch criteria (N2). The possibility of unrestricted reuse or use in covered embankments is constrained as the leaching of some contaminants exceeds the Dutch criteria (N1) and Finland criteria (F2), respectively.
Based on the assessment of batch and column leaching results with different standards, it can be inferred that IBA can be reused in paved applications or earthworks subjected to minimum infiltration. Soil Quality Decree (2007) suggested that either bentonite mat, HDPE film, or sand bentonite polymer gel can be used to reduce the infiltration when IBA is used in field applications. Moreover, these standards have recommended some location-specific requirements (see Table S3 for details), which should also be adhered to before field applications of IBA.
Conclusions
The present study characterizes IBA from two MSW incineration plants in Delhi and presents a systematic approach for understanding the effect of particle size, L/S, and pH on the contaminant leaching from IBA. The major findings of the study are:
-
Silica (quartz) is the primary constituent of IBA followed by CaO (calcite) and Al2O3 (feldspar).
-
GSM fraction of IBA is appreciably different from OM and SLM as it is observed to have highest percentage of SiO2, lowest content of CaO, Cl and SO3 and least amount of organics and trace metal(loid)s among the three fractions.
-
Batch leaching at intrinsic pH (i.e., DBL) reveals GSM to be least contaminated, while SLM is the most contaminated among the three IBA fractions. The reduction in leaching of contaminants with an increase in particle size of IBA is attributable to greater concentration of contaminants in the finer fractions of IBA.
-
Column leaching (i.e., DCL) results reveal rapid dissolution of Cd, Cu, Ni, Zn, and Cl− at lower L/S ratio followed by insignificant release at higher L/S. However, the release of Mo, Sb, and \({\text{SO}}_{4}^{2-}\) increases steadily with an increase in L/S.
-
pH-dependent leaching (i.e., PBL) demonstrates significant influence of pH on leaching of Cd, Pb, and Zn. The variation in the leaching concentration of chlorides and sulfates due to fluctuations in pH is minimal in comparison with those observed in trace metal(loid)s.
-
IBA is suitable for disposal in non-hazardous waste landfills regardless of particle size at its intrinsic pH (10–12). However, a reduced pH of IBA (8–10) due to weathering reactions can restrict its disposal to non-hazardous waste landfills due to aggravated leaching of antimony.
-
It is recommended to dispose IBA in monofils as co-disposal with MSW in sanitary landfills can cause the pH of IBA to shift toward the acidic range, which may exacerbate the contaminant leaching from IBA.
-
It is further revealed that IBA can be reused in paved roads or earthworks subjected to minimum infiltration. Moreover, the location-specific requirements (see Table S3) as recommended in reuse standards should also be adhered to before field applications of IBA.
In India, handling IBA is relatively new and has no established guidelines. Thus, this paper provides insight into the leaching characteristics of IBA and provides recommendations that would assist stakeholders and policymakers to develop guidelines before disposal or reuse of IBA. The present study is limited to the experimental investigation of IBA; however, it is suggested to perform a comparative environmental assessment of IBA with conventional materials using life cycle assessment prior to its field applications.
Data availability
All data supporting the finding of work will be made available on a reasonable request to corresponding author.
References
Åberg A, Kumpiene J, Ecke H (2006) Evaluation and prediction of emissions from a road built with bottom ash from municipal solid waste incineration (MSWI). Sci Total Environ 355:1–12. https://doi.org/10.1016/j.scitotenv.2005.03.007
Ahmed FN, Lan CQ (2012) Treatment of landfill leachate using membrane bioreactors: a review. Desalination 287:41–54. https://doi.org/10.1016/j.desal.2011.12.012
Ai H, Clavier KA, Watts BE et al (2019) The efficacy of pH-dependent leaching tests to provide a reasonable estimate of post-carbonation leaching. J Hazard Mater 373:204–211. https://doi.org/10.1016/j.jhazmat.2019.03.089
Alam Q, Schollbach K, van Hoek C et al (2019) In-depth mineralogical quantification of MSWI bottom ash phases and their association with potentially toxic elements. Waste Manag 87:1–12. https://doi.org/10.1016/j.wasman.2019.01.031
APHA, AWWA, WEF (2012) Standard Methods for the Examination of Water and Wastewater. Washington, DC, USA
Arickx S, Van Gerven T, Vandecasteele C (2006) Accelerated carbonation for treatment of MSWI bottom ash. J Hazard Mater 137:235–243. https://doi.org/10.1016/j.jhazmat.2006.01.059
Arm M (2004) Variation in deformation properties of processed MSWI bottom ash: results from triaxial tests. Waste Manag 24:1035–1042. https://doi.org/10.1016/j.wasman.2004.07.013
Blanc D, Gonzalez L, Lupsea-Toader M, de Brauer C (2018) Mineralogical evolution and leaching behaviour of a heap of bottom ash as a function of time: influence on its valorization. Waste Biomass Valoriz 9:2517–2527. https://doi.org/10.1007/s12649-018-0444-1
Blasenbauer D, Huber F, Lederer J et al (2020) Legal situation and current practice of waste incineration bottom ash utilisation in Europe. Waste Manag 102:868–883. https://doi.org/10.1016/j.wasman.2019.11.031
Brouwer P (2006) Theory of XRF: getting acquainted with the principles. PANalytical B.V. https://www.chem.purdue.edu/xray/docs/Theory%20of%20XRF.pdf. Accessed 20 Feb 23
Bruder-Hubscher V, Lagarde F, Leroy MJF et al (2001) Utilisation of bottom ash in road construction: evaluation of the environmental impact. Waste Manag Res 19:545–556. https://doi.org/10.1177/0734242x0101900611
BS 1377-3 (2018) Methods of test for soils for civil engineering purposes- Part 3: Chemical and electrochemical tests. British Standards, UK
BS EN 12457-2 (2002) Characterisation of waste- Leaching Compliance test for leaching of granular waste materials and sludges Part 2: One stage batch test at a liquid to solid ratio of 10 l/kg for materials with particle size below 4 mm (without or with size reduction). British Standard, UK
BS EN 12457-4 (2002) Characterisation of waste- Leaching Compliance test for leaching of granular waste materials and sludges Part 4: One stage batch test at a liquid to solid ratio of 10 l/kg for materials with particle size below 10 mm (without or with size reduction). British Standard, UK.
BS EN 14429 (2015) Characterization of waste - Leaching behaviour test—Influence of pH on leaching with initial acid/base addition. British Standard, UK.
BS EN 14405 (2017) Characterization of Waste—Leaching Behaviour Test—Up-flow Percolation Test (under specified conditions). British Standard, UK.
Bureau EI (2005) Reference document on the best available techniques for waste incineration. European Union, Brussels, Belgium.
Caviglia C, Confalonieri G, Corazzari I et al (2019) Effects of particle size on properties and thermal inertization of bottom ashes (MSW of Turin’s incinerator). Waste Manag 84:340–354. https://doi.org/10.1016/j.wasman.2018.11.050
Chang FY, Wey Y (2006) Comparison of the characteristics of bottom and fly ashes generated from various incineration processes. J Hazard Mater 138:594–603. https://doi.org/10.1016/j.jhazmat.2006.05.099
Chang HL, Jau WC, Li KC, Lin CF (2004) The evaluation of the feasibility of utilizing incineration bottom ash as subbase material. Environ Inform Arch 2:1033–1047
Chimenos JM, Segarra M, Fernández MA, Espiell F (1999) Characterization of the bottom ash in municipal solid waste incinerator. J Hazard Mater 64:211–222. https://doi.org/10.1016/S0304-3894(98)00246-5
CPCB (2021) Annual report on implementation of solid waste management rules, 2016. Central Pollution Control Board, New Delhi, India. https://cpcb.nic.in/uploads/MSW/MSW_AnnualReport_2020-21.pdf. Accessed 20 Feb 23
Cornelis G, Van Gerven T, Vandecasteele C (2006) Antimony leaching from uncarbonated and carbonated MSWI bottom ash. J Hazard Mater 137:1284–1292. https://doi.org/10.1016/j.jhazmat.2006.04.048
DEFRA (2013) Incineration of Municipal Solid Waste. Department for Environment, Food & Rural Affairs, UK
del Valle-Zermeño R, Formosa J, Chimenos JM et al (2013) Aggregate material formulated with MSWI bottom ash and APC fly ash for use as secondary building material. Waste Manag 33:621–627. https://doi.org/10.1016/j.wasman.2012.09.015
Di Gianfilippo M, Hyks J, Verginelli I et al (2018) Leaching behaviour of incineration bottom ash in a reuse scenario: 12years-field data vs. lab test results. Waste Manag 73:367–380. https://doi.org/10.1016/j.wasman.2017.08.013
Dijkstra JJ, van der Sloot HA, Comans RNJ (2006) The leaching of major and trace elements from MSWI bottom ash as a function of pH and time. Appl Geochem 21:335–351. https://doi.org/10.1016/j.apgeochem.2005.11.003
Dou X, Ren F, Nguyen MQ et al (2017) Review of MSWI bottom ash utilization from perspectives of collective characterization, treatment and existing application. Renew Sustain Energy Rev 79:24–38. https://doi.org/10.1016/j.rser.2017.05.044
EU Council (2003) Council decision of 19 December 2002 establishing criteria and procedures for the acceptance of waste at landfills pursuant to Article 16 of and Annex II to Directive 1999/31/EC. The Council of the European Union. https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2003:011:0027:0049:EN:PDF, last accessed 2022/08/10. Accessed 20 Feb 23
Ginés O, Chimenos J, Vizcarro A et al (2009) Combined use of MSWI bottom ash and fly ash as aggregate in concrete formulation: environmental and mechanical considerations. J Hazard Mater 169:643–650. https://doi.org/10.1016/j.jhazmat.2009.03.141
Gori M, Bergfeldt B, Pfrang-Stotz G et al (2011) Effect of short-term natural weathering on MSWI and wood waste bottom ash leaching behaviour. J Hazard Mater 189:435–443. https://doi.org/10.1016/j.jhazmat.2011.02.045
Government of Finland (2017) Government Decree on the recovery of certain wastes in earth construction (843/2017). Ministry of the Environment, Finland. https://www.finlex.fi/en/laki/kaannokset/2017/en20170843%0A. Accessed 20 Feb 23
Government of French Republic (2011) Arrêté du 18 novembre 2011 relatif au recyclage en technique routière des mâchefers d’incinération de déchets non dangereux. The Ministry of Ecology, Sustainable Development, Transport and Housing, France. https://www.legifrance.gouv.fr/loda/id/JORFTEXT000024873229/2020-11-20/. Accessed 20 Feb 23
Government of Wallonia (2001) Arrêté du Gouvernement wallon favorisant la valorisation de certains déchets, dated 14/06/2001 (latest modification on 05/07/2018). Wallonia, Belgium. http://environnement.wallonie.be/legis/dechets/decat024.htm. Accessed 20 Feb 23
Grathwohl P, Susset B (2009) Comparison of percolation to batch and sequential leaching tests: theory and data. Waste Manag 29:2681–2688. https://doi.org/10.1016/j.wasman.2009.05.016
Gupta G, Datta M, Ramana GV, Alappat BJ (2021a) MSW incineration bottom ash (MIBA) as a substitute to conventional materials in geotechnical applications: a characterization study from India and comparison with literature. Constr Build Mater 308:124925. https://doi.org/10.1016/j.conbuildmat.2021.124925
Gupta G, Datta M, Ramana GV et al (2021b) Contaminants of concern (CoCs) pivotal in assessing the fate of MSW incineration bottom ash (MIBA): first results from India and analogy between several countries. Waste Manag 135:167–181. https://doi.org/10.1016/j.wasman.2021.08.036
Hjelmar O, Holm J, Crillesen K (2007) Utilisation of MSWI bottom ash as sub-base in road construction: first results from a large-scale test site. J Hazard Mater 139:471–480. https://doi.org/10.1016/j.jhazmat.2006.02.059
Holm O, Simon FG (2017) Innovative treatment trains of bottom ash (BA) from municipal solid waste incineration (MSWI) in Germany. Waste Manag 59:229–236. https://doi.org/10.1016/j.wasman.2016.09.004
Huber F, Blasenbauer D, Aschenbrenner P, Fellner J (2019) Chemical composition and leachability of differently sized material fractions of municipal solid waste incineration bottom ash. Waste Manag 95:593–603. https://doi.org/10.1016/j.wasman.2019.06.047
Hyks J, Astrup T (2009) Influence of operational conditions, waste input and ageing on contaminant leaching from waste incinerator bottom ash: a full-scale study. Chemosphere 76:1178–1184. https://doi.org/10.1016/j.chemosphere.2009.06.040
Hyks J, Nesterov I, Mogensen E et al (2011) Leaching from waste incineration bottom ashes treated in a rotary kiln. Waste Manag Res 29:995–1007. https://doi.org/10.1177/0734242X11417490
Intrakamhaeng V, Clavier KA, Roessler JG, Townsend TG (2019) Limitations of the toxicity characteristic leaching procedure for providing a conservative estimate of landfilled municipal solid waste incineration ash leaching. J Air Waste Manag Assoc 69:623–632. https://doi.org/10.1080/10962247.2019.1569172
ISWA (2013) Waste-to-Energy State-of-the-Art-Report Statistics, sixth edition with revision. International Solid Waste Association. https://www.nswai.org/docs/ISWA6_7-000-2_WtE_State_of_the_Art_Report_2012_Revised_November_2013.pdf. Accessed 20 Feb 23
Izquierdo M, Querol X, Josa A et al (2008) Comparison between laboratory and field leachability of MSWI bottom ash as a road material. Sci Total Environ 389:10–19. https://doi.org/10.1016/j.scitotenv.2007.08.020
Kalbe U, Berger W, Eckardt J, Simon F-G (2008) Evaluation of leaching and extraction procedures for soil and waste. Waste Manag 28:1027–1038. https://doi.org/10.1016/j.wasman.2007.03.008
Kaza S, Yao LC, Bhada-Tata P, Van Woerden F (2018) What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050. World Bank, Washington, DC, USA. https://doi.org/10.1596/978-1-4648-1329-0. Accessed 20 Feb 23
Keppert M, Pavlík Z, Tydlitát V et al (2012) Properties of municipal solid waste incineration ashes with respect to their separation temperature. Waste Manag Res 30:1041–1048. https://doi.org/10.1177/0734242X12448513
Kosson DS, van der Sloot HA, Garrabrants AC, Seignette PFAB (2014) Leaching test relationships, laboratory-to-field comparisons and recommendations for leaching evaluation using the leaching environmental assessment framework (LEAF). Report by United States Environmental Protection Agency, USA
Kulikowska D, Klimiuk E (2008) The effect of landfill age on municipal leachate composition. Bioresour Technol 99:5981–5985. https://doi.org/10.1016/j.biortech.2007.10.015
Lam CHK, Ip AWM, Barford JP, McKay G (2010) Use of incineration MSW ash: a review. Sustainability 2:1943–1968
Le NH, Razakamanantsoa A, Nguyen ML et al (2018) Evaluation of physicochemical and hydromechanical properties of MSWI bottom ash for road construction. Waste Manag 80:168–174. https://doi.org/10.1016/j.wasman.2018.09.007
Li L, Youneng T, Tarek A et al (2019) Characterization of leachates from landfills containing MSW-I residues. J Hazardous Toxic Radioact Waste 23:4019013. https://doi.org/10.1061/(ASCE)HZ.2153-5515.0000451
Lidelöw S, Lagerkvist A (2007) Evaluation of leachate emissions from crushed rock and municipal solid waste incineration bottom ash used in road construction. Waste Manag 27:1356–1365. https://doi.org/10.1016/j.wasman.2006.07.021
Lin WY, Heng KS, Sun X, Wang JY (2015) Accelerated carbonation of different size fractions of MSW IBA and the effect on leaching. Waste Manag 41:75–84. https://doi.org/10.1016/j.wasman.2015.04.003
Loginova E, Volkov DS, van de Wouw PMF et al (2019) Detailed characterization of particle size fractions of municipal solid waste incineration bottom ash. J Clean Prod 207:866–874. https://doi.org/10.1016/j.jclepro.2018.10.022
Lynn CJ, Ghataora GS, Dhir RK (2016) Environmental impacts of MIBA in geotechnics and road applications. Environ Geotech 5:31–55
Malik M, Soni NK, Kv K et al (2016) Characterisation of fly ash from coal-fired thermal power plants using energy dispersive X-ray fluorescence spectrometry. Sci Rev Chem Commun 6(4):91–101
Margallo M, Aldaco R, Irabien Á (2014) Environmental management of bottom ash from municipal solid waste incineration based on a life cycle assessment approach. Clean Technol Environ Policy 16:1319–1328. https://doi.org/10.1007/s10098-014-0761-4
Meima JA, Comans RNJ (1999) The leaching of trace elements from municipal solid waste incinerator bottom ash at different stages of weathering. Appl Geochem 14:159–171. https://doi.org/10.1016/S0883-2927(98)00047-X
Minane JR, Becquart F, Abriak NE, Deboffe C (2017) Upgraded mineral sand fraction from MSWI bottom ash: an alternative solution for the substitution of natural aggregates in concrete applications. Procedia Eng 180:1213–1220. https://doi.org/10.1016/j.proeng.2017.04.282
MoEF&CC (2020) Enforcement and monitoring guidelines for sand mining. Ministry of Environment, Forest and Climate Change, New Delhi, India http://environmentclearance.nic.in/writereaddata/SandMiningManagementGuidelines2020.pdf. Accessed 20 Feb 23
Ng WPQ, Lam HL, Varbanov PS, Klemeš JJ (2014) Waste-to-Energy (WTE) network synthesis for Municipal Solid Waste (MSW). Energy Convers Manag 85:866–874. https://doi.org/10.1016/j.enconman.2014.01.004
Nikravan M, Ramezanianpour AA, Maknoon R (2020) Study on physiochemical properties and leaching behavior of residual ash fractions from a municipal solid waste incinerator (MSWI) plant. J Environ Manag 260:110042. https://doi.org/10.1016/j.jenvman.2019.110042
Oehmig WN, Roessler JG, Blaisi NI, Townsend TG (2015) Contemporary practices and findings essential to the development of effective MSWI ash reuse policy in the United States. Environ Sci Policy 51:304–312. https://doi.org/10.1016/j.envsci.2015.04.024
Olsson S, Gustafsson JP, Berggren Kleja D et al (2009) Metal leaching from MSWI bottom ash as affected by salt or dissolved organic matter. Waste Manag 29:506–512. https://doi.org/10.1016/j.wasman.2008.03.017
Polettini A, Pomi R, Carcani G (2005) The effect of Na and Ca salts on MSWI bottom ash activation for reuse as a pozzolanic admixture. Resour Conserv Recycl 43:403–418. https://doi.org/10.1016/j.resconrec.2004.07.004
Priti, Mandal K (2019) Review on evolution of municipal solid waste management in India: practices, challenges and policy implications. J Mat Cycl Waste Manag 21(6): 1263–1279
Quina MJ, Bordado JCM, Quinta-Ferreira RM (2009) The influence of pH on the leaching behaviour of inorganic components from municipal solid waste APC residues. Waste Manag 29:2483–2493. https://doi.org/10.1016/j.wasman.2009.05.012
Quina MJ, Bordado JCM, Quinta-Ferreira RM (2011) Percolation and batch leaching tests to assess release of inorganic pollutants from municipal solid waste incinerator residues. Waste Manag 31:236–245. https://doi.org/10.1016/j.wasman.2010.10.015
Rendek E, Ducom G, Germain P (2007) Assessment of MSWI bottom ash organic carbon behavior: a biophysicochemical approach. Chemosphere 67:1582–1587. https://doi.org/10.1016/j.chemosphere.2006.11.054
Saikia N, Mertens G, Van Balen K et al (2015) Pre-treatment of municipal solid waste incineration (MSWI) bottom ash for utilisation in cement mortar. Constr Build Mater 96:76–85. https://doi.org/10.1016/j.conbuildmat.2015.07.185
Santos RM, Mertens G, Salman M et al (2013) Comparative study of ageing, heat treatment and accelerated carbonation for stabilization of municipal solid waste incineration bottom ash in view of reducing regulated heavy metal/metalloid leaching. J Environ Manag 128:807–821. https://doi.org/10.1016/j.jenvman.2013.06.033
SDGs (2018) Sustainable development goals homepage. https://sdgs.un.org/goals. Accessed 20 Feb 23
Shen P, Zheng H, Lu J, Poon CS (2021) Utilization of municipal solid waste incineration bottom ash (IBA) aggregates in high-strength pervious concrete. Resour Conserv Recycl 174:105736
Soil Quality Decree (2007) Regeling Bodemkwaliteit. VROM, Ruimte en Milieu, Ministerie van Volkshuisvesting, Ruimtelijke Ordeling en Milieubeheer, Den Haag. https://rwsenvironment.eu/subjects/soil/legislation-and/soil-quality-decree/. Accessed 20 Feb 23
SWM Rules (2016) Solid waste management rules. Ministry of Environment, Forest and Climate Change, New Delhi, India. https://cpcb.nic.in/uploads/MSW/SWM_2016.pdf. Accessed 20 Feb 23
Song GJ, Kim KH, Seo YC, Kim SC (2004) Characteristics of ashes from different locations at the MSW incinerator equipped with various air pollution control devices. Waste Manag 24:99–106. https://doi.org/10.1016/S0956-053X(03)00073-4
Sormunen LA, Rantsi R (2015) To fractionate municipal solid waste incineration bottom ash: key for utilisation? Waste Manag Res 33:995–1004. https://doi.org/10.1177/0734242X15600052
Tan ST, Ho WS, Hashim H et al (2015) Energy, economic and environmental (3E) analysis of waste-to-energy (WTE) strategies for municipal solid waste (MSW) management in Malaysia. Energy Convers Manag 102:111–120. https://doi.org/10.1016/j.enconman.2015.02.010
Tang P, Florea MVA, Spiesz P, Brouwers HJH (2015) Characteristics and application potential of municipal solid waste incineration (MSWI) bottom ashes from two waste-to-energy plants. Constr Build Mater 83:77–94. https://doi.org/10.1016/j.conbuildmat.2015.02.033
Tang P, Florea MVA, Spiesz P, Brouwers HJH (2016) Application of thermally activated municipal solid waste incineration (MSWI) bottom ash fines as binder substitute. Cem Concr Compos 70:194–205. https://doi.org/10.1016/j.cemconcomp.2016.03.015
Traina G, Morselli L, Adorno GP (2007) Electrokinetic remediation of bottom ash from municipal solid waste incinerator. Electrochim Acta 52:3380–3385. https://doi.org/10.1016/j.electacta.2006.05.067
Tripathy U (2018) A 21st Century Vision on Waste to Energy in India: A Win-Win Strategy for Energy Security and Swachh Bharat Mission (Clean India Mission). A report by the United Nations Centre for Regional Development in collaboration with the Ministry of Housing and Urban Affairs (MoHUA), Government of India and Ministry of the Environment, Government of Japan, India. https://www.uncrd.or.jp/content/documents/6717FINAL-Background%20paper_Tripathy.pdf. Accessed 20 Feb 23
USEPA 3050 (1986) Acid digestion of sediment, sludge and soils. Test Methods for Evaluating Soil-Waste SW-846. United States Environmental protection agency, Cincinnati, OH, USA
USEPA 40 CFR 261.24 (2011) Toxicity Characteristic. United States Environmental protection agency, Cincinnati, OH, USA. https://www.govinfo.gov/content/pkg/CFR-2011-title40-vol26/pdf/CFR-2011-title40-vol26-sec261-24.pdf. Accessed 20 Feb 23
USEPA 1313 (2013) Leaching Test (Liquid–Solid Partitioning as a Function of Extract pH) of Inorganic Species in Solid Materials Using a Parallel Batch Extraction Test. United States Environmental protection agency, Cincinnati, OH, USA
USEPA 1314 (2013) Leaching Test (Liquid–Solid Partitioning as a Function of Liquid–Solid Ratio) of Constituents in Solid Materials Using an Upflow Percolation Column. United States Environmental protection agency, Cincinnati, OH, USA
Van Fan Y, Jiang P, Klemeš JJ et al (2021) Integrated regional waste management to minimise the environmental footprints in circular economy transition. Resour Conserv Recycl 168:105292. https://doi.org/10.1016/j.resconrec.2020.105292
Vaitkus A, Grazulyte J, Vorobjovas V et al (2018) Potential of MSWI bottom ash to be used as aggregate in road building materials. Balt J Road Bridg Eng 13:77–86. https://doi.org/10.3846/bjrbe.2018.401
van de Wouw PMF, Loginova E, Florea MVA, Brouwers HJH (2020) Compositional modelling and crushing behaviour of MSWI bottom ash material classes. Waste Manag 101:268–282. https://doi.org/10.1016/j.wasman.2019.10.013
van der Sloot HA (1996) Developments in evaluating environmental impact from utilization of bulk inert wastes using laboratory leaching tests and field verification. Waste Manag 16:65–81. https://doi.org/10.1016/S0956-053X(96)00028-1
Van Gerven T, Van Keer E, Arickx S et al (2005) Carbonation of MSWI-bottom ash to decrease heavy metal leaching, in view of recycling. Waste Manag 25:291–300. https://doi.org/10.1016/j.wasman.2004.07.008
Wagner TP, Raymond T (2015) Landfill mining: Case study of a successful metals recovery project. Waste Manag 45:448–457. https://doi.org/10.1016/j.wasman.2015.06.034
Xuan D, Poon CS (2018) Removal of metallic Al and Al/Zn alloys in MSWI bottom ash by alkaline treatment. J Hazard Mater 344:73–80. https://doi.org/10.1016/j.jhazmat.2017.10.002
Yao J, Li WB, Kong Q et al (2012) Effect of weathering on the mobility of zinc in municipal solid waste incinerator bottom ash. Fuel 93:99–104. https://doi.org/10.1016/j.fuel.2011.11.026
Yin K, Dou X, Ren F et al (2018) Statistical comparison of leaching behavior of incineration bottom ash using seawater and de-ionized water: significant findings based on several leaching methods. J Hazard Mater 344:635–648. https://doi.org/10.1016/j.jhazmat.2017.11.004
Zhang H, He PJ, Shao LM, Li XJ (2008) Leaching behavior of heavy metals from municipal solid waste incineration bottom ash and its geochemical modeling. J Mater Cycles Waste Manag 10:7–13. https://doi.org/10.1007/s10163-007-0191-z
Acknowledgements
The support and assistance provided by officials of M/s Timarpur Okhla Waste Management Company Limit, M/s Delhi MSW Solutions Ltd., and South Delhi Municipal Corporation for sample collection are gratefully acknowledged.
Funding
The authors have no relevant financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Contributions
Deepesh Bansal was involved in conceptualization, methodology, and writing—original draft; Garima Gupta contributed to reviewing and editing; GV Ramana and Manoj Datta were involved in supervision, reviewing and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bansal, D., Gupta, G., Ramana, G.V. et al. Environmentally responsible disposal and reuse of MSW incineration bottom ash: assessment from two Indian plants. Clean Techn Environ Policy 26, 1439–1454 (2024). https://doi.org/10.1007/s10098-023-02550-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-023-02550-y