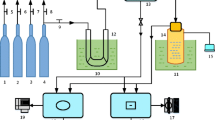
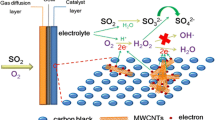
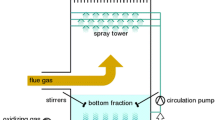
Abstract
Emissions reduction or conversion by clean technologies has always been a mission for environmental protection and sustainable development. A green, electrochemically driven conversion of SO2 to NaHSO4 from flue gas by pH variation was conducted. Experiments were carried out to investigate some factors affecting the conversion process, such as O2 function in flue gas, selected initial pH (pH0) of solution, and CO2 effects of high concentration. Experimental results revealed that (i) O2 was essential for SO2 absorption, since the pH of solution was decreased more effectively than that of flue gas without O2. The lowest pH of absorption solution with pouring O2 was about 3.2, which was benefit to formatting HSO3 − but hindering absorption of CO2. Meanwhile, O2 from flue gas accelerated the adsorption of SO2 for its oxidability to change HSO3 − and SO3 2− ions into SO4 2−. (ii) At the conditions of different pH0, the minimum absorption efficiency of SO2 was 89.3 %, corresponding to pH0 5.0 and 7.0, while maximum value was 100.1 % corresponding to pH0 6.0, which meant pH0 6.0 was more appropriate for SO2 conversion by electrochemistry under the experimental pH0 range. (iii) The absorption efficiency of CO2 was at a very low level under conditions of pH0 5.0, 6.0, and 7.0, whose value of minimum and maximum was 0.07 and 0.19 %. The result showed that high concentration of CO2 from flue gas had little affection on desulfurization under the experimental pH0 range, which enable to infer that byproduct of NaHSO4 is pure enough.




Similar content being viewed by others
References
Foo D, Lam HL (2013) Green technologies for Asia-Pacific economies. Clean Technol Environ Policy 15:674
Gutiérrez Ortiz FJ, Vidal F, Ollero P, Salvador L, Cortés V (2006) Pilot-plant technical assessment of wet flue gas desulfurization using limestone. Ind Eng Chem Res 45:1466–1477
Happel M, Luckas N, Vines F, Sobota M, Laurin M, Gorling A, Libuda J (2011) SO2 adsorption on Pt(111) and oxygen precovered Pt(111): a combined Infrared Reflection Absorption Spectroscopy and density functional study. J Phys Chem C 115:479–491
Heldebrant DJ, Koech PK, Yonker CR (2010) A reversible zwitterionic SO2-binding organic liquid. Energy Environ Sci 3:111–113
Hu GX, Sun ZG, Gao HY (2010) Novel process of simultaneous removal of SO2 and NO2 by sodium humate solution. Environ Sci Technol 44:6712–6717
Kikkawa H, Nakamoto T, Morishita M, Yamada K (2002) New wet FGD process using granular limestone. Ind Eng Chem Res 41:3028–3036
Lee KY, Kim CS, Kim HG, Cheong MS, Mukherjee DK, Jung KD (2010) Effects of halide anions to absorb SO2 in ionic liquids. Bull Korean Chem Soc 31:1937–1940
Li C, Zhang Q, Krotkov NA, Streets DG, He K, Tsay SC, Gleason JF (2010) Recent large reduction in sulfur dioxide emissions from Chinese power plants observed by the Ozone Monitoring Instrument. Geophys Res Lett 37:1–6
Liu H, Wang C, Li Xuan XL, Jiang CC, Cui HN (2007) A novel electro-Fenton process forwater treatment: reaction-controlled pH adjustment and performance assessment. Environ Sci Technol 41:2937–2942
Matsushima N, Li Y, Nishioka M, Sadakata M, Qi H, Xu X (2004) Novel dry-desulfurization process using Ca(OH)2/fly ash sorbent in a circulating fluidized bed. Environ Sci Technol 38:6867–6874
Michalski JA (2006) Equilibria in limestone-based FGD process: magnesium addition. Ind Eng Chem Res 45:1945–1954
Ren SH, Hou YC, Wu WZ, Liu QY, Xiao YF, Chen XT (2010) Properties of ionic liquids absorbing SO2 and the mechanism of the absorption. J Phys Chem B 114:2175–2179
Sekhavatjou MS, Hosseini Alhashemi A, Karbassi AR, Daemolzekr E (2011) Minimization of air pollutants emissions by process improvement of catalytic reforming unit in an Iranian old refinery. Clean Technol Environ Policy 13:743–749
Smith SJ, van Aardenne J, Klimont Z, Andres RJ, Volke A, Delgado Arias S (2010) Anthropogenic sulfur dioxide emissions: 1850–2005. Atmos Chem Phys Discuss 10:16111–16151
Sumathi S, Bhatia S, Lee KT, Mohamed AR (2009) Optimization of microporouspalm shell activated carbon production for flue gas desulphurization: experimental and statistical studies. Bioresour Technol 100:1614–1621
Sun ZG, Zhao Y, Gao HY, Hu GX (2010) Removal of SO2 from flue gas by sodium humate solution. Energy Fuel 24:1013–1019
Tursun H, Li ZY, Liu R, Li Y, Wang XH (2015) Contribution weight of engineering technology on pollutant emission reduction based on IPAT and LMDI methods. Clean Technol Environ Policy 17:225–235
Vidal BF, Ollero P, Gutierrez Ortiz FJ, Arjona R (2005) Catalytic oxidation of S(IV) in seawater slurries of activated carbon. Environ Sci Technol 39:5031–5036
Wang C, Liu H, Li XZ, Shi J, Ouyang G, Peng M, Jiang C, Cui H (2008) A new concept of desulfurization: the electrochemically driven and green conversion of SO2 to NaHSO4 in aqueous solution. Environ Sci Technol 42:8585–8590
Wang CG, Cui GK, Luo XY, Xu YJ, Li HR, Dai S (2011) Highly efficient and reversible SO2 capture by tunable Azole-based ionic liquids through multiple-site chemical absorption. J Am Chem Soc 133:11916–11919
Xu Y (2011) Improvements in the operation of SO2 scrubbers in China’s coal power plants. Environ Sci Technol 45:380–385
Zhi YT, Zheng JJ, Zhou YP, Sun Y, Su W, Zhou L (2011) Studies on the enhancement of flue gas desulfurization with oxidation reaction. Energy Fuel 25:5038–5043
Zhu HS, Mao YP, Yang XJ, Yu C, Long XL, Yuan WK (2010) Simultaneous absorption of NO and SO2 into Fe(II)–EDTA solution coupled with the Fe(II)–EDTA regeneration catalyzed by activated carbon. Sep Purif Technol 74:1–6
Acknowledgments
This work was supported by Beijing Sinen En-Tech Co., Ltd. and National Natural Science Foundation of China (Grant No. 21476051), as well as the Science and Technology Project of Guangdong Province (Grant No. 2013B010403028). The anonymous reviewers are acknowledged for their helpful comments to improve the article quality.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, L., Guo, J., Yang, Z. et al. Green SO2 conversion from flue gas by pH variation. Clean Techn Environ Policy 18, 593–600 (2016). https://doi.org/10.1007/s10098-015-1034-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-015-1034-6