Abstract
Urinary tract infections (UTIs) are among the most common bacterial infections in men and urine culture is gold standard for diagnosis. Considering the high prevalence of culture-negative specimens, any method that identifies such specimens is of interest. The aim was to evaluate a new screening concept for flow cytometry analysis (FCA). The outcomes were evaluated against urine culture, uropathogen species and three conventional screening methods. A prospective, consecutive study examined 1,312 urine specimens, collected during January and February 2012. The specimens were analyzed using the Sysmex UF1000i FCA. Based on the FCA data culture negative specimens were identified in a new model by use of linear discriminant analysis (FCA-LDA). In total 1,312 patients were included. In- and outpatients represented 19.6% and 79.4%, respectively; 68.3% of the specimens originated from women. Of the 610 culture-positive specimens, Escherichia coli represented 64%, enterococci 8% and Klebsiella spp. 7%. Screening with FCA-LDA at 95% sensitivity identified 42% (552/1312) as culture negative specimens when UTI was defined according to European guidelines. The proposed screening method was either superior or similar in comparison to the three conventional screening methods. In conclusion, the proposed/suggested and new FCA-LDA screening method was superior or similar to three conventional screening methods. We recommend the proposed screening method to be used in clinic to exclude culture negative specimens, to reduce workload, costs and the turnaround time. In addition, the FCA data may add information that enhance handling and support diagnosis of patients with suspected UTI pending urine culture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urinary tract infections (UTIs) are among the most common infections in community and hospitalized patients with more than 175 million UTI incidences worldwide [1]. UTI is caused by pathogenic microorganisms which induce infection and an inflammatory response with presence of leukocyturia and erythrocyturia. However, UTIs are often harmless and self-eradicated [2]. Nevertheless, UTIs are associated with high morbidity and costs and especially among patients with diabetes [3]. In the United States UTIs account annually for more than 7 million physician visits, more than 1 million emergency department visits and more than 100,000 hospitalizations. The estimated annual cost for treatment is calculated to more than US $1 billion and the indirect cost estimated to approximately $1.6 billion [4, 5]. Furthermore, 15% of all antibiotics prescribed to outpatients and data from some European countries also suggest a similar rate [6, 7].
In a large German study on patients with diabetes mellitus type 2 the cost was estimated to €316 per UTI event with a total increased cost of €3916 per patient in the UTI group compared with the non UTI group [3].
For diagnosis of UTI symptoms such as urgency, dysuria, frequent urination, back pain, leukocyturia and a positive nitrite test are considered reliable indicators [2, 8]. However, urine culture is the gold standard for diagnosis of UTI but is laborious and moderately costly, with a turnaround time of 24 to 48 h [9].
In clinical laboratories, urine specimens are among the most commonly encountered and approximately a quarter to more than half are considered culture negative [9, 10]. For this reason, any screening method that identifies and excludes urine specimens with non-significant bacteriuria would be of great interest [11–13].
Flow cytometry analysis (FCA) is a promising method to identify and enumerate bacteria, leukocytes, erythrocytes and other particles in urine. The second generation automated FCA instrument, the Sysmex UF-1000i (Medical Electronics, Kobe, Japan) has a separate detection channel for bacteria with improved sensitivity (SE) and specificity (SP) [11–15].
Using FCA, we have recently evaluated the inflammatory response of leukocytes and erythrocytes in urine for different pathogens in patients with suspected UTI [9]. Based on the dataset we now aimed to set up and assess a new screening model to identify and rule out culture negative urine specimens in patients with suspected UTI prior to culture. The prerequisites for the screening model are high SE to prevent specimens with significant bacteriuria (SBU) from being erroneously classified as negative (i.e. false negatives) and a relatively high SP to prevent unnecessary culturing. The present screening model was evaluated against three conventional screening methods [11, 16, 17].
Material and methods
Collection of urine specimens
A prospective consecutive multicenter study was conducted during January and February 2012 which analyzed urine specimens from in- and outpatients. The specimens were collected in non-preservative tubes, stored and transported at ≤6 °C to the Department of Clinical Microbiology at the University Hospital of Umeå for analysis. All specimens were from the county of Västerbotten, Sweden.
Urinalysis
All specimens underwent FCA and urine culture within 3 h of arrival to the laboratory. Specimens that arrived after 4 PM were analyzed with flow cell analysis (FCA) and stored at +6 °C until cultured the following morning. Excluded were specimens from pregnant women, urinary catheter and those that lacked complete FCA data.
Flow cytometry analysis was performed using the UF-1000i instrument (Sysmex, TOA Medical Electronics, Kobe, Japan), supported by the Sysmex software version of 00–15 [9]. The screening model was built on the observed bacterial and leukocyte counts.
Urine culture
Gram-negative and Gram-positive uropathogens were identified by Brilliance™ UTI agar (Oxoid Ltd., Basingstoke, UK) biochemical tests, and urine culture was performed as previously described [9].
Species identification
Isolates were identified in specimens with presence of ≥106 colony forming units/L (CFU/L) and those with mixed flora (with both gram negative and gram positive bacteria) with a dominating pathogen (i.e. bacterial count at least 10 times higher than any other species) [9].
Significant bacteriuria
SBU was defined in accordance with European guidelines (at ≥106 CFU/L of an uropathogen with acute uncomplicated cystitis) [7] and was the gold standard in the present article [18, 19]. In patients with <108 (≥106 and 107 CFU/L) and where the presence or absence of UTI symptoms could not be determined, the specimens were classified indeterminant.
Evaluations were also done when SBU was defined as ≥107 or ≥108 CFU/L of a uropathogen, respectively, irrespective of presence of UTI symptoms.
Ethical approval
All procedures were in accordance with institutional and national ethical standards and the Helsinki declaration.
Statistical analysis
Urine specimens analyzed with FCA and urine culture were used to derive a decision rule that based on FCA data determines which specimens should be cultured. Screening methods based on bacterial counts (BC) and white blood cell counts (WBC) have been suggested by Jolkkonen, Manoni and De Rosa [11, 16, 17]. Here, specimens are cultured if either BC > a or WBC > b, where a and b are the method specific cut-off values: Jolkonnen: a = 405, b = 16; Manoni: a = 125, b = 40; De Rosa: a = 170, b = 150.
We suggest the FCA-LDA approach that uses linear discriminant analysis (LDA) on the FCA variables BC and WBC [20]. The FCA-LDA rule was derived using data from specimens that were concluded to be significant bacteriuria or non-significant bacteriuria and with BC < 5000. A LDA-model was fitted to the log-transformed FCA-values, assuming homoscedasticity and proportional priors, which resulted in a decision rule: [ln (BC) > α + βln(WBC)]. The intercept α was tuned so that the rule had the desired sensitivity (SE). The decision rule is a line, where specimens above the line are cultured (Fig. 1).
Plot of 1,312 urine specimens with significant, non-significant and indeterminant bacteriuria and the classification criteria or the different screening methods examined. Specimens above the classification line were predicted as positives and send to culture. P (blue color) = “positive specimens", i.e. specimens with significant bacteriuria when urinary tract infection was defined according to European guidelines. N (red colour) = “negative specimens", i.e. specimens with non-significant bacteriuria when urinary tract infection was defined according to European guidelines. Q (green colour) = “indeterminant/questionable” specimens, i.e. specimens with indeterminant significant bacteriuria when urinary tract infection was defined according to European guidelines (mainly specimens that lacked information regarding UTI symptoms), number of bacteria (Bact = Y-axis) and white blood cells (WBC = X- axis) estimated by flow cell instrument per uL. Red lines represent the cut off for linear discriminant analysis at: 98% sensitivity (FCA-LDA98), 95% sensitivity (FCA-LDA95) and 90% sensitivity (FCA-LDA90). Dark blue color represents the cut-off values according to De Rosa et al. (cut off: bacteria 170, white blood cells 150). Green color represents the cut-off values according to Manoni et al. (cut off: bacteria 125, white blood cells 40). Light blue color represents the cut-off values according to Jolkkonen et al. (cut off: bacteria 405, white blood cells 16)
The methods were evaluated in terms of their SE and specificity (SP), negative and positive predictive values (PPV and NPV), the number of cultured specimens and the relative cost (RC) compared to the standard procedure. RC was calculated under the assumption that the running cost of culture is five times as high as running FCA. The evaluations were performed for different definitions of UTI.
In a similar style as described above a LDA-model, based on BC and the variable B-FSC (the forward scatter / “particle size”), was derived to determine if the specimens’ bacteria were gram positive or gram negative. This resulted in a classification rule: [ln (BC) > α + β ln(B-FSC)] where specimens were predicted gram positive if the inequality was valid and gram-negative otherwise.
Results
In total, 1587 urine specimens were eligible for FCA and urine culture. Two hundred seventy-five specimens (17.3%) were excluded: 125 urinary catheters, 128 specimens from pregnant women, 18 that lacked clinical information and four specimens with candidauria. In total, 1312 specimens were enrolled in the present study (Fig. 1).
Among genders, 68.3% (p < 0.001) of the specimens originated from women with a mean age of 58.9 years (standard deviation 25.5) versus 31.7% and 61.7 years (20.8) for men. Outpatients represented 79.4% and inpatients 20.6%. The demographic information of the dataset is presented in Table 1.
When UTI was defined according to European guidelines, 36.0% (473/1312 specimens) had significant bacteriuria, 56.5% (741/1312 specimens) had non significant bacteriuria and 7.5% (98/1312 specimens) were classified as indeterminant, due to lack of clinical information.
E. coli was the most predominant uropathogen, representing 71.2% (337/473) of the uropathogens followed by Enterococcus faecalis 6.8%, Klebsiella pneumoniae 6.1% and coagulase-negative staphylococci 5.1% (Tables 1 and 2).
FCA-LDA decision rules, using BC and WBC as the predictors and controlling the SE at 90%, 95% and 98%, respectively, were derived, i.e.
-
FCA-LDA98: ln(BC) > 10.46–0.65 ln (WBC)
-
FCA-LDA95: ln(BC) > 8.22–0.65 ln (WBC)
-
FCA-LDA90: ln(BC) > 7.07–0.65 ln (WBC)
Recall that a specimen is cultured if the inequality is fulfilled. The FCA-LDA-rules were compared to the bivariate decision rules suggested by Jolkkonen, Manoni and De Rosa [11, 16, 17]. The six decision rules and their outcome are shown in Fig. 1.
Evaluation when SBU was defined according to European guidelines
Overall, when the FCA-LDA98 decision rule was used and UTI was defined according to European guidelines (at ≥106 CFU/L of an uropathogen with acute uncomplicated cystitis) [7] then the screen resulted in 98% SE (464 true positive specimens), 52% SP (385 true negative specimens) and 32% (421 specimens) were identified as culture negative (Fig. 2, total data). The corresponding numbers for the more restrictive inclusion rules FCA-LDA95 and FCA-LDA90 were (95%, 65%, and 42%) and (90%, 83%, and 56%), respectively (Fig. 2, Table 2). In comparison, the screening methods by Jolkkonen, Manoni and De Rosa had similar SE as for FCA-LDA98; however, the Jolkkonen’s and Manoni’s methods had lower SP (41%) compared to FCA-LDA98 (52%) and De Rosa (51%). The outcome of the six decision rules are presented in Table 2. In general, the screening methods had relative high SE for E. coli (0–3% above average SE for the examined methods) and Klebsiella/Citrobacter groups (KC-group 0–5% above average SE). Relative low SE was found for the enterococci group (EN group −1 to −18% below average SE) and the streptococci group (SR group −18 to −30% below average SE for the examined methods ; Table 2).
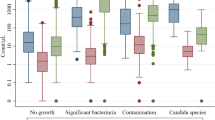
Outcome of the 1312 urine specimens examined by flow cytometry analysis and samples sent to culture after screening using linear discriminant analysis at 90, 95 and 98% sensitivity when significant bacteriuria was defined according to European guidelines. Blue arrows: represent the outcome of linear discriminant analysis at 98, 95 and 90% sensitivity, respectively (FCA-LDA98, FCA-LDA95, FCA-LDA90) when urinary tract infection was defined according to European guidelines. G+ = gram positive species. G– = gram negative species. 1SBU = significant bacteriuria, blue-colored boxes; Q-SBU = indeterminant (questionable) SBU, green-colored boxes; Non SBU, red-colored boxes. 2Number of specimens send to culture when FCA-LDA selection was applied. 3 Specimens send to culture was classified as Gram negative or Gram positive bacteria
Screening at SBU defined according to European guidelines yielded higher SE and SP than UTI defined at ≥107 and ≥108 /CFU/L (Table 3).
At FCA-LDA98, FCA- LDA95 and FCA- LDA90 screening 9, 23 (9 + 14) and 47 (23 + 24) specimens proved false negative, respectively, representing 0.7, 1.7 and 3.6% of the specimens. Information related to the false negative specimens is presented in Table 4. For the FCA-LDA95 screen, seven of the false negative specimens had 108 CFU/L, eight 106 and 108 CFU/L each. Patients 2, 3, 7, 9, 10, 16 had all low WBC- and elevated bacterial counts indicating asymptomatic bacteriuria in these patients. Patient 8 had “high” WBC and red blood cell (RBC) counts but low bacterial counts despite presence of Proteus mirabilis at 108 CFU/L at culture. Of the 47 false negative specimens, 72% (34/47) originated from women.
Evaluation when SBU was defined at ≥107 and ≥108 CFU/L
Overall, defining UTI at ≥107 CFU/L of an uropathogen, irrespective of presence of UTI symptoms, resulted in lower sensitivities (3–9%) and similar specificities in comparison when SBU was defined according to European guidelines (Tables 3 and 5). Defining UTI as ≥108 CFU/L (irrespective of presence of UTI symptoms) resulted in higher sensitivities (1–5%) and lower specificities (2–4%) in comparison to European guidelines (Tables 3 and 6).
Also at these screening methods SE had great impact on the proportion of specimens identified as culture negative (26–56%; Tables 2, 3, 5, and 6). For all methods a higher SE was observed for E. coli (0–5% above average SE), and lower SE for the enterococci (0 to −18% below average SE) and streptococci groups (0 to −25%) (Tables 2, 5, and 6).
Prediction of gram groups
Bacteria were predicted to their gram group according to their FCA results such that ln (BC) > −8.49 + 3.82 ln (B-FSC) were classified to be gram-positive otherwise to be gram-negative. In specimens sent to culture, 74–80% of the bacteria were correctly classified to their gram group (Fig. 2).
Calculation of the relative cost in a model including FCA screening found a 5–36% cost reduction compared with non-selective culture depending on the screening method used (Table 3). Implementation of the FCA-LDA95 estimated a 22% cost reduction when UTI was defined according to European guidelines (Table 3).
Discussion
UTIs are a common infection and one of the most commonly analyzed specimens in clinical microbiological laboratories. The aim of the present study was to present and evaluate a new model for identification of culture negative urine specimens identified by FCA.
The basis of the new screening model was to establish the cut off for bacterial and leukocyte counts based on LDA which differs from conventional screening models based on fixed cut off’s for bacteria- and WBC counts (Fig. 1) [11–13, 15–17, 20–22]. The new model was evaluated with respect to: SE, definitions of significant bacteriuria, different uropathogens, group of uropathogens, mixed flora and culture negative specimens and three conventional screening methods [12, 16, 17]. At evaluation the new LDA method proved superior to the Jolkkonen and Manoni methods but similar to that described by De Rosa et al. [17]. The prerequisite for the presented screening method was high SE since diagnosis was later confirmed by urine culture. Since the outcome for SBU defined according to European guidelines was superior compared with 107 CFU/L, irrespective of UTI symptoms. For this reason is the European guidelines recommended.
Currently, the instrument is mainly used for screening to identify and rule out culture negative specimens. However, screening can be done at different levels of SE, e.g. at lower SE for non hospitalized patients as UTIs are often harmless and self eradicating [2] or at higher SE (or omitted) in high risk patients in intensive care, immunosuppressed patients, pregnancy and those with pyelonephritis. In these patients, urine culture is recommended irrespective of the outcome of the FCA screening to avoid missed diagnosis in those deemed high risk. Since Streptococcus agalactiae is a potential threat to the unborn child and mother, we always recommend urine culture at pregnancy to identify colonization at low colony counts (<106 CFU/L).
Overall, we found that FCA-LDA95 provided a good balance between high SE and specimens excluded as culture negative (> 40%). At our laboratory, nearly 12,600 (of 30,000) specimens could be excluded from culture providing a significant reduction in workload, costs and turnaround time. The predicted cost reduction in the present model (5–36%) is in accordance with others [23]. In addition, FCA analysis adds important information to clinicians in identifying or rejecting patients for antimicrobial treatment. For those treated, the microorganisms Gram group adds further information for treatment success.
The microorganism’s Gram stain identity was predicted by use of B-FSC [24, 25] with an accuracy of 76% (74–80%) in the present study. Rod-shaped bacteria are predicted at higher accuracy than for cocci, >90% and 29%, respectively [26]. Use of NaOH-sodium dodecyl sulfate (SDS) has been proven to facilitate discrimination between Gram positive and Gram negative bacteria [27].
Excluded specimens (urinary catheters and those from pregnant women) were re-examined with a similar outcome as those included (data not shown). LDA screening seems therefore also to be useful for these specimens but further studies are warranted.
Of Among the false negative specimens patient 8 had “high” WBC counts and low bacterial counts despite presence of Proteus mirabilis at 108 CFU/L at culture. We speculate that the bacterial FCA-staining may have failed in this specimen.
The strength of the present study is the new screening approach, the large cohort and the comprehensive evaluations and against three conventional screening methods [11, 16, 17]. Also the outcomes are presented for different uropathogens, groups of bacteria, negative cultures and pinpoint the strength and weakness in the different methods as the impact of the definition of SBU.
Similar evaluations are, to our knowledge, not reported for SBU defined according to the European definition (at ≥106 CFU/L of an uropathogen with acute uncomplicated cystitis) [7]. In addition we also evaluated the outcome at ≥107 and ≥108 CFU/L irrespective of presence of UTI symptoms.
The distribution of uropathogens was in accordance with other studies [20, 28, 29] and sampling was carried out to minimize the destruction of RBC and WBC [30]. Also, the sampling approach possessed a very low risk for contamination with improved specimen quality.
A weakness is the cohort’s population structure with respect to gender, age, in−/outpatients, type of specimens, etc., which differs from other studies and explains the differences in outcome [11–13, 15–17, 19–22, 31]. However, the pathophysiology of UTI is expected to be quite similar despite some differences between species [9] and cohorts. However, the relatively large differences in outcome between studies is probably due to different cut off’s rather than differences between cohorts, which is supported in the present study where similar outcome was found for the present and De Rosa’s screening model. Also at an optimal cut off we expect a similar outcome, unless one method is superior. If so, a universal cut off can be implicated which significantly enhances implementation of the FCA screening method in clinic, but further studies are warranted.
Also, the delay from initiation until publication is a weakness but the distribution of uropathogens causing UTI is quite conserved over decades [2, 20, 28, 29, 32].
The present cohort may not reflect urine specimens from an average population but rather a selected cohort of specimens from patients with suspected UTI, pyelonephritis and failure or control after treatment. However, the specimens are representative of the urine samples analyzed at our laboratory.
Flow cytometry is a promising method to identify and rule out culture negative urine specimens prior to culture [11–13, 15–17, 19–22, 31]. The present screening model can be recommended in clinic for pre-screening of UTI specimens prior to culture to improve laboratory management of UTI specimens and service to clinicians. By an early identification of culture negative specimens, patients are excluded for unnecessary antimicrobial treatment and its associated side effects.
In summary, a new screening concept is presented based on LDA of FCA data that excludes 42% as culture negative urine specimens prior to culture when UTI was defined according to European guidelines. The present LDA screening method was superior or similar to three conventional methods and is recommended for clinical use to reduce workloads, costs, turnaround time and time to diagnosis.
Change history
15 July 2017
An erratum to this article has been published.
References
Stamm WE, Hooton TM (1993) Management of urinary tract infections in adults. N Engl J Med 329(18):1328–1334
Ferry SA, Holm SE, Stenlund H, Lundholm R, Monsen TJ (2004) The natural course of uncomplicated lower urinary tract infection in women illustrated by a randomized placebo controlled study. Scand J Infect Dis 36(4):296–301
Wilke T, Bottger B, Berg B, Groth A, Botteman M, Yu S, Fuchs A, Maywald U (2016) Healthcare burden and costs associated with urinary tract infections in type 2 diabetes mellitus patients: an analysis based on a large sample of 456,586 German patients. Nephron 132(3):215–226
Mazzulli T (2002) Resistance trends in urinary tract pathogens and impact on management. J Urol 168(4 Pt 2):1720–1722
Foxman B (2002) Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med 113(Suppl 1A):5S–13S
The Medical Products Agency (2007) UTI – lower urinary tract infections in females, 18 edn. The Medical Products Agency, Stockholm, pp 8–45
Grabe M, Bartoletti R, Bjerklund Johansen TE, Cai T, Çek M, Köves B, Naber KG, Pickard RS, Tenke P, Wagenlehner F, Wullt B (2015) Guidelines on urological infections. European Association of Urology, p 1–86
Bent S, Nallamothu BK, Simel DL, Fihn SD, Saint S (2002) Does this woman have an acute uncomplicated urinary tract infection? JAMA 287(20):2701–2710
Monsen T, Ryden P (2015) Flow cytometry analysis using Sysmex UF-1000i classifies uropathogens based on bacterial, leukocyte, and erythrocyte counts in urine specimens among patients with urinary tract infections. J Clin Microbiol 53(2):539–545
Okada H, Sakai Y, Kawabata G, Fujisawa M, Arakawa S, Hamaguchi Y, Kamidono S (2001) Automated urinalysis – evaluation of the Sysmex UF-50. Am J Clin Pathol 115(4):605–610
Jolkkonen S, Paattiniemi EL, Karpanoja P, Sarkkinen H (2010) Screening of urine samples by flow cytometry reduces the need for culture. J Clin Microbiol 48(9):3117–3121
Broeren MA, Bahceci S, Vader HL, Arents NL (2011) Screening for urinary tract infection with the Sysmex UF-1000i urine flow cytometer. J Clin Microbiol 49(3):1025–1029
Pieretti B, Brunati P, Pini B, Colzani C, Congedo P, Rocchi M, Terramocci R (2010) Diagnosis of bacteriuria and leukocyturia by automated flow cytometry compared with urine culture. J Clin Microbiol 48(11):3990–3996
Marschal M, Wienke M, Hoering S, Autenrieth IB, Frick JS (2012) Evaluation of 3 different rapid automated systems for diagnosis of urinary tract infections. Diagn Microbiol Infect Dis 72(2):125–130
Wang J, Zhang Y, Xu D, Shao W, Lu Y (2010) Evaluation of the Sysmex UF-1000i for the diagnosis of urinary tract infection. Am J Clin Pathol 133(4):577–582
Manoni F, Fornasiero L, Ercolin M, Tinello A, Ferrian M, Hoffer P, Valverde S, Gessoni G (2009) Cutoff values for bacteria and leukocytes for urine flow cytometer Sysmex UF-1000i in urinary tract infections. Diagn Microbiol Infect Dis 65(2):103–107
De Rosa R, Grosso S, Bruschetta G, Avolio M, Stano P, Modolo ML, Camporese A (2010) Evaluation of the Sysmex UF1000i flow cytometer for ruling out bacterial urinary tract infection. Clin Chim Acta 411(15–16):1137–1142
Kouri T, Fogazzi G, Gant V, Hallander H, Hofmann W, Guder WG (2000) European urinalysis guidelines. Scandinavian Journal of Clinical ánd Laboratory Investigation 60(supplement 231):1–96
Aspevall O, Hallander H, Gant V, Kouri T (2001) European guidelines for urinalysis: a collaborative document produced by European clinical microbiologists and clinical chemists under ECLM in collaboration with ESCMID. Clin Microbiol Infect 7(4):173–178
Brilha S, Proenca H, Cristino JM, Hanscheid T (2010) Use of flow cytometry (Sysmex) UF-100 to screen for positive urine cultures: in search for the ideal cut-off. Clin Chem Lab Med 48(2):289–292
Dai Q, Jiang Y, Shi H, Zhou W, Zhou S, Yang H (2014) Evaluation of the automated urine particle analyzer UF-1000i screening for urinary tract infection in nonpregnant women. Clin Lab 60(2):275–280
de Frutos-Serna M, Asensio-Calle ML, Haro-Perez AM, Blazquez-de Castro AM, Gutierrez-Zufiaurre MN, Iglesias-Garcia J (2014) Evaluation of the Sysmex UF-1000i flow cytometer for screening of urinary tract infection. Enferm Infecc Microbiol Clin 32(3):147–151
Ilki A, Ayas R, Ozsoy S, Soyletir G (2017) Cost-effectiveness of a new system in ruling out negative urine cultures on the day of administration. Eur J Clin Microbiol Infect Dis. Jan 22 [Epub ahead of print]
Gessoni G, Saccani G, Valverde S, Manoni F, Caputo M (2015) Does flow cytometry have a role in preliminary differentiation between urinary tract infections sustained by gram positive and gram negative bacteria? An Italian polycentric study. Clin Chim Acta 440:152–156
Jimenez-Guerra G, Heras-Canas V, Valera-Arcas MD, Rodriguez-Granger J, Navarro JM, Gutierrez-Fernandez J (2017) Comparison between urine culture profile and morphology classification using fluorescence parameters of the Sysmex UF-1000i urine flow cytometer. J Appl Microbiol 122(2):473–480
Geerts N, Jansz AR, Boonen KJ, Wijn RP, Koldewijn EL, Boer AK, Scharnhorst V (2015) Urine flow cytometry can rule out urinary tract infection, but cannot identify bacterial morphologies correctly. Clin Chim Acta 448:86–90
Wada A, Kono M, Kawauchi S, Takagi Y, Morikawa T, Funakoshi K (2012) Rapid discrimination of gram-positive and gram-negative bacteria in liquid samples by using NaOH-sodium dodecyl sulfate solution and flow cytometry. PLoS One 7(10):e47093
Aspevall O, Hallander H (2000) Reference methodology for laboratory diagnostics at clinical bacteriological laboratories. I. Infectious diagnostics. I 5. Urinary tract infections/bacteriuria, 2nd edn. Swedish Institute for Infectious Disease Control, Stockholm
Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ (2015) Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol 13(5):269–284
Kouri T, Vuotari L, Pohjavaara S, Laippala P (2002) Preservation of urine for flow cytometric and visual microscopic testing. Clin Chem 48(6 Pt 1):900–905
Ackermann RJ, Monroe PW (1996) Bacteremic urinary tract infection in older people. J Am Geriatr Soc 44(8):927–933
Gupta K, Sahm DF, Mayfield D, Stamm WE (2001) Antimicrobial resistance among uropathogens that cause community-acquired urinary tract infections in women: a nationwide analysis. Clin Infect Dis 33(1):89–94
Acknowledgements
We acknowledge Ulf Hammar Department of Biostatistics, Karolinska Institute Stockholm and Tamara Matti Department of Infectious Diseases, University Hospital of Umeå for support and registration of datasets; Tore Carlsson from Sysmex, Sweden for support, calibration and installation of the instrument; and Neil Williamson for linguistic revision.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study provided financial support was through regional agreement between Umeå University and Västerbotten County Council on cooperation in the field of Medicine, Odontology and Health.
Conflict of interest
The authors declare no conflict of interest.
Ethics approval
The article was conducted without participation of human or animals.
Informed consent
This study did not involve human subjects or samples; therefore, no informed consent is required.
Additional information
The original version of this article was revised: The original version of this article unfortunately contains an error in the abstract.
An erratum to this article is available at https://doi.org/10.1007/s10096-017-3059-8.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Monsen, T., Ryden, P. A new concept and a comprehensive evaluation of SYSMEX UF-1000i flow cytometer to identify culture-negative urine specimens in patients with UTI. Eur J Clin Microbiol Infect Dis 36, 1691–1703 (2017). https://doi.org/10.1007/s10096-017-2964-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-017-2964-1