Abstract
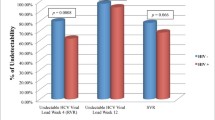
It is commonly accepted that human immunodeficiency (HIV) coinfection negatively impacts on the rates of sustained virological response (SVR) to therapy with pegylated interferon plus ribavirin (PR). However, this hypothesis is derived from comparing different studies. The aim of this study was to determine the impact of HIV coinfection on SVR to PR in one single population. In a multicentric, prospective study conducted between 2000 and 2013, all previously naïve hepatitis C virus (HCV)-infected patients who started PR in five Spanish hospitals were analyzed. SVR was evaluated 24 weeks after the scheduled end of therapy. Of the 1046 patients included in this study, 413 (39 %) were coinfected with HIV. Three hundred and forty-one (54 %) HCV-monoinfected versus 174 (42 %) HIV/HCV-coinfected patients achieved SVR (p < 0.001). The corresponding figures for undetectable HCV RNA at treatment week 4 were 86/181 (47 %) versus 59/197 (30 %), p < 0.001. SVR was observed in 149 (69 %) HCV genotype 2/3-monoinfected subjects versus 91 (68 %) HIV/HCV genotype 2/3-coinfected subjects (p = 0.785). In the HCV genotype 1/4-infected population, 188 (46 %) monoinfected patients versus 82 (30 %) with HIV coinfection (p < 0.001) achieved SVR. In this subgroup, absence of HIV coinfection was independently associated with higher SVR [adjusted odds ratio (95 % confidence interval): 2.127 (1.135–3.988); p = 0.019] in a multivariate analysis adjusted for age, sex, baseline HCV RNA load, IL28B genotype, fibrosis stage, and type of pegylated interferon. HIV coinfection impacts on the rates of SVR to PR only in HCV genotype 1/4-infected patients, while it has no effect on SVR in the HCV genotype 2/3-infected subpopulation.



Similar content being viewed by others

References
World Health Organization (WHO) (2014) Guidelines for the screening, care and treatment of persons with hepatitis C infection. Available online at: http://apps.who.int/iris/bitstream/10665/111747/1/9789241548755_eng.pdf?ua=1&ua=1. Accessed October 2014
Neukam K, Camacho A, Caruz A et al (2012) Prediction of response to pegylated interferon plus ribavirin in HIV/hepatitis C virus (HCV)-coinfected patients using HCV genotype, IL28B variations, and HCV-RNA load. J Hepatol 56:788–794
Pineda JA, Caruz A, Di Lello FA et al (2011) Low-density lipoprotein receptor genotyping enhances the predictive value of IL28B genotype in HIV/hepatitis C virus-coinfected patients. AIDS 25:1415–1420
Fried MW, Shiffman ML, Reddy KR et al (2002) Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 347:975–982
Manns MP, McHutchison JG, Gordon SC et al (2001) Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 358:958–965
Torriani FJ, Rodriguez-Torres M, Rockstroh JK et al (2004) Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med 351:438–450
Carrat F, Bani-Sadr F, Pol S et al (2004) Pegylated interferon alfa-2b vs standard interferon alfa-2b, plus ribavirin, for chronic hepatitis C in HIV-infected patients: a randomized controlled trial. JAMA 292:2839–2848
Soriano V, Sulkowski M, Bergin C et al (2002) Care of patients with chronic hepatitis C and HIV co-infection: recommendations from the HIV-HCV International Panel. AIDS 16:813–828, Review
[No authors listed] (2002) NIH Consensus Statement on Management of Hepatitis C: 2002. NIH Consens State Sci Statements 19:1–46, Review
Guideline of the European Association for the Study of the Liver (EASL) (2014) Home page at: http://www.easl.eu. Accessed October 2014
Guideline of the European AIDS Clinical Society (EACS) (2014) Home page at: http://www.eacsociety.org/. Accessed October 2014
Scheuer PJ (1991) Classification of chronic viral hepatitis: a need for reassessment. J Hepatol 13:372–374
Castéra L, Vergniol J, Foucher J et al (2005) Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology 128:343–350
Tural C, Galeras JA, Planas R et al (2008) Differences in virological response to pegylated interferon and ribavirin between hepatitis C virus (HCV)-monoinfected and HCV-HIV-coinfected patients. Antivir Ther 13:1047–1055
Mira JA, Rivero A, de Los Santos-Gil I et al (2012) Hepatitis C virus genotype 4 responds better to pegylated interferon with ribavirin than genotype 1 in HIV-infected patients. AIDS 26:1721–1724
Odolini S, Amadasi S, Cerini C et al (2014) Sustained virological response to peginterferon therapy in patients infected with HCV (genotypes 2 and 3), with or without HIV. BMC Infect Dis 14:S4
Neukam K, García-Rey S, Cifuentes C et al (2012) HIV-coinfection leads to a modest increase in plasma HCV-RNA load in patients with chronic HCV infection. Antiviral Res 95:212–215
Acknowledgments
This work was supported by the Red de Investigación en SIDA (ISCIII-RETIC RD06/006 and ISCIII-RETIC RD12/0017) and the Consejería de Salud of the Junta de Andalucía (grant numbers PI-0492/2012 and AC-0095-2013). K.N. is the recipient of a Miguel Servet research grant from the Instituto de Salud Carlos III (grant number CP13/00187). J.A.P. is the recipient of an intensification grant from the Instituto de Salud Carlos III (grant number Programa-I3SNS).
Conflict of interest
A.C.-I. has received speaker honorarium from Janssen, Gilead, and AbbVie laboratories. She has lead consultation activities to AbbVie, Gilead, Janssen, and Merck laboratories, and has taken part as advisory committees to AbbVie, ViiH, and Janssen. K.N. has received lecture fees from Janssen-Cilag, Roche, Bristol-Meyers Squibb, and Merck Sharp & Dohme, and has received research support from Janssen-Cilag, Bristol-Meyers Squibb, Merck Sharp & Dohme, Gilead Sciences, and Abbott Pharmaceuticals. J.A.P. reports having received consulting fees from GlaxoSmithKline, Bristol-Myers Squibb, Abbott Pharmaceuticals, Gilead, Merck Sharp & Dohme, Schering-Plough, Janssen-Cilag, and Boehringer Ingelheim. He has received research support from GlaxoSmithKline, Roche, Bristol-Myers Squibb, Schering-Plough, Abbott Pharmaceuticals, and Boehringer Ingelheim, and has received lecture fees from GlaxoSmithKline, Roche, Abbott Pharmaceuticals, Bristol-Myers Squibb, Gilead, Merck Sharp & Dohme, Janssen-Cilag, Boehringer Ingelheim, and Schering-Plough. A.R. has received consultancy fees from Abbott Laboratories, Bristol-Myers Squibb, Boehringer Ingelheim, ViiV Healthcare, and Gilead Sciences. M.D.M. has received speaker and/or consulting fees from Gilead Sciences, Bristol, Janssen, and AbbVie. J.M. has been an investigator in clinical trials supported by Roche, Bristol-Myers Squibb, and Abbott Pharmaceuticals. He has received lecture fees from Roche, Gilead, Boehringer Ingelheim, and Bristol-Myers Squibb, and consulting fees from Boehringer Ingelheim, Bristol Myers-Squibb, and Merck Sharp & Dome.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical committee of Valme Hospital and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Monje-Agudo, P., Castro-Iglesias, A., Rivero-Juárez, A. et al. Impact of HIV infection on sustained virological response to treatment against hepatitis C virus with pegylated interferon plus ribavirin. Eur J Clin Microbiol Infect Dis 34, 1929–1936 (2015). https://doi.org/10.1007/s10096-015-2434-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-015-2434-6