Abstract
Torque teno virus (TTV) and torque teno mini virus (TTMV) have been potentially related to liver diseases. The aim of the study was to quantify TTV and TTMV in human immunodeficiency virus (HIV)/hepatitis C virus (HCV)-coinfected patients to study the relationship between the TTV and TTMV viral loads and the severity of liver disease. We carried out a cross-sectional study in 245 patients coinfected with HIV and HCV (HIV/HCV-group), 114 patients monoinfected with HIV (HIV-group), and 100 healthy blood donors (Control-group). Plasma samples were tested for TTV and TTMV by quantitative real-time polymerase chain reaction (PCR). The prevalences of TTV and TTMV infections in the HIV/HCV-group and the HIV-group were significantly higher than the Control-group (p < 0.05). Furthermore, TTV and TTMV coinfections were found in 92.2 % (226/245) in the HIV/HCV-group, 84.2 % (96/114) in the HIV-group, and 63 % (63/100 %) in the Control-group (p ≤ 0.05). HIV/HCV-coinfected patients with HIV viral load ≥50 copies/mL and patients with severe activity grade had the highest viral loads of TTV and TTMV (p ≤ 0.05). HIV/HCV-coinfected patients with high TTV load (>2.78 log copies/μL) had increased odds of having advanced fibrosis or severe necroinflammatory activity grade in the liver biopsy. Moreover, HIV/HCV-coinfected patients with high TTMV load (>1.88 log copies/μL) had decreased odds of having no/minimal fibrosis and no/mild activity grade, and increased odds of having a high fibrosis progression rate. In conclusion, TTV and TTMV might play a role in the development of liver disease in immunodeficiency patients, such as the patients coinfected with HIV and HCV.



Similar content being viewed by others
References
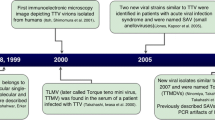
Nishizawa T, Okamoto H, Konishi K, Yoshizawa H, Miyakawa Y, Mayumi M (1997) A novel DNA virus (TTV) associated with elevated transaminase levels in posttransfusion hepatitis of unknown etiology. Biochem Biophys Res Commun 241(1):92–97
Takahashi K, Iwasa Y, Hijikata M, Mishiro S (2000) Identification of a new human DNA virus (TTV-like mini virus, TLMV) intermediately related to TT virus and chicken anemia virus. Arch Virol 145(5):979–993
Huang LY, Øystein Jonassen T, Hungnes O, Grinde B (2001) High prevalence of TT virus-related DNA (90%) and diverse viral genotypes in Norwegian blood donors. J Med Virol 64(3):381–386
Takahashi K, Hoshino H, Ohta Y, Yoshida N, Mishiro S (1998) Very high prevalence of TT virus (TTV) infection in general population of Japan revealed by a new set of PCR primers. Hepatol Res 12:233–239
Okamoto H (2009) History of discoveries and pathogenicity of TT viruses. Curr Top Microbiol Immunol 331:1–20
Davidson F, MacDonald D, Mokili JLK, Prescott LE, Graham S, Simmonds P (1999) Early acquisition of TT virus (TTV) in an area endemic for TTV infection. J Infect Dis 179(5):1070–1076
Ball JK, Curran R, Berridge S, Grabowska AM, Jameson CL, Thomson BJ, Irving WL, Sharp PM (1999) TT virus sequence heterogeneity in vivo: evidence for co-infection with multiple genetic types. J Gen Virol 80(Pt 7):1759–1768
Sugiyama K, Goto K, Ando T, Mizutani F, Terabe K, Kawabe Y, Wada Y (1999) Route of TT virus infection in children. J Med Virol 59(2):204–207
Simmonds P, Davidson F, Lycett C, Prescott LE, MacDonald DM, Ellender J, Yap PL, Ludlam CA, Haydon GH, Gillon J, Jarvis LM (1998) Detection of a novel DNA virus (TTV) in blood donors and blood products. Lancet 352(9123):191–195
Shibayama T, Masuda G, Ajisawa A, Takahashi M, Nishizawa T, Tsuda F, Okamoto H (2001) Inverse relationship between the titre of TT virus DNA and the CD4 cell count in patients infected with HIV. AIDS 15(5):563–570
Christensen JK, Eugen-Olsen J, Sorensen M, Ullum H, Gjedde SB, Pedersen BK, Nielsen JO, Krogsgaard K (2000) Prevalence and prognostic significance of infection with TT virus in patients infected with human immunodeficiency virus. J Infect Dis 181(5):1796–1799
Okamoto H, Nishizawa T, Ukita M (1999) A novel unenveloped DNA virus (TT virus) associated with acute and chronic non-A to G hepatitis. Intervirology 42(2–3):196–204
Focosi D, Maggi F, Albani M, Macera L, Ricci V, Gragnani S, Di Beo S, Ghimenti M, Antonelli G, Bendinelli M, Pistello M, Ceccherini-Nelli L, Petrini M (2010) Torquetenovirus viremia kinetics after autologous stem cell transplantation are predictable and may serve as a surrogate marker of functional immune reconstitution. J Clin Virol 47(2):189–192
Kaufmann GR, Elzi L, Weber R, Furrer H, Giulieri S, Vernazza P, Bernasconi E, Hirschel B, Battegay M; Swiss HIV Cohort Study (2011) Interruptions of cART limits CD4 T-cell recovery and increases the risk for opportunistic complications and death. AIDS 25(4):441–451
Madsen CD, Eugen-Olsen J, Kirk O, Parner J, Kaae Christensen J, Brasholt MS, Ole Nielsen J, Krogsgaard K (2002) TTV viral load as a marker for immune reconstitution after initiation of HAART in HIV-infected patients. HIV Clin Trials 3(4):287–295
Okamoto H, Ukita M, Nishizawa T, Kishimoto J, Hoshi Y, Mizuo H, Tanaka T, Miyakawa Y, Mayumi M (2000) Circular double-stranded forms of TT virus DNA in the liver. J Virol 74(11):5161–5167
Okamoto H, Nishizawa T, Kato N, Ukita M, Ikeda H, Iizuka H, Miyakawa Y, Mayumi M (1998) Molecular cloning and characterization of a novel DNA virus (TTV) associated with posttransfusion hepatitis of unknown etiology. Hepatol Res 10(1):1–16
Kao JH, Chen W, Chen PJ, Lai MY, Chen DS (2000) TT virus infection in patients with chronic hepatitis B or C: Influence on clinical, histological and virological features. J Med Virol 60(4):387–392
Charlton M, Adjei P, Poterucha J, Zein N, Moore B, Therneau T, Krom R, Wiesner R (1998) TT-virus infection in North American blood donors, patients with fulminant hepatic failure, and cryptogenic cirrhosis. Hepatology 28(3):839–842
Ikeda H, Takasu M, Inoue K, Okamoto H, Miyakawa Y, Mayumi M (1999) Infection with an unenveloped DNA virus (TTV) in patients with acute or chronic liver disease of unknown etiology and in those positive for hepatitis C virus RNA. J Hepatol 30(2):205–212
Tokita H, Murai S, Kamitsukasa H, Yagura M, Harada H, Takahashi M, Okamoto H (2002) High TT virus load as an independent factor associated with the occurrence of hepatocellular carcinoma among patients with hepatitis C virus-related chronic liver disease. J Med Virol 67(4):501–509
Zein NN, Arslan M, Li H, Charlton MR, Gross JB Jr, Poterucha JJ, Therneau TM, Kolbert CP, Persing DH (1999) Clinical significance of TT virus infection in patients with chronic hepatitis C. Am J Gastroenterol 94(10):3020–3027
Moriyama M, Matsumura H, Shimizu T, Shioda A, Kaneko M, Miyazawa K, Miyata H, Tanaka N, Uchida T, Arakawa Y (2001) Histopathologic impact of TT virus infection on the liver of type C chronic hepatitis and liver cirrhosis in Japan. J Med Virol 64(1):74–81
Alvarez do Barrio M, González Díez R, Hernández Sánchez JM, Oyonarte Gómez S (2005) Residual risk of transfusion-transmitted viral infections in Spain, 1997–2002, and impact of nucleic acid testing. Euro Surveill 10(2):20–22
Jacobs WH, Goldberg SB, Balint JA, Boyce HW, Browning TH, Cooper JN, Earnest DL, Friedman E, Greene M, Mellow MA, Pitt HA, Todaro JL; Patient Care Committee of the American Gastroenterological Association (1989) Statement on outpatient percutaneous liver biopsy. Dig Dis Sci 34(3):322–323
Bedossa P, Poynard T (1996) An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology 24(2):289–293
Moen EM, Sleboda J, Grinde B (2002) Real-time PCR methods for independent quantitation of TTV and TLMV. J Virol Methods 104(1):59–67
Biagini P, Gallian P, Touinssi M, Cantaloube JF, Zapitelli JP, de Lamballerie X, de Micco P (2000) High prevalence of TT virus infection in French blood donors revealed by the use of three PCR systems. Transfusion 40(5):590–595
Maggi F, Bendinelli M (2009) Immunobiology of the Torque teno viruses and other anelloviruses. Curr Top Microbiol Immunol 331:65–90
Griffiths P (1999) Time to consider the concept of a commensal virus? Rev Med Virol 9(2):73–74
Zhong S, Yeo W, Tang MW, Lin XR, Mo F, Ho WM, Hui P, Johnson PJ (2001) Gross elevation of TT Virus genome load in the peripheral blood mononuclear Cells of cancer patients. Ann N Y Acad Sci 945:84–92
Touinssi M, Gallian P, Biagini P, Attoui H, Vialettes B, Berland Y, Tamalet C, Dhiver C, Ravaux I, De Micco P, De Lamballerie X (2001) TT virus infection: prevalence of elevated viraemia and arguments for the immune control of viral load. J Clin Virol 21(2):135–141
Thom K, Petrik J (2007) Progression towards AIDS leads to increased torque teno virus and torque teno minivirus titers in tissues of HIV infected individuals. J Med Virol 79(1):1–7
Mariscal LF, López-Alcorocho JM, Rodríguez-Iñigo E, Ortiz-Movilla N, de Lucas S, Bartolomé J, Carreño V (2002) TT virus replicates in stimulated but not in nonstimulated peripheral blood mononuclear cells. Virology 301(1):121–129
Reiberger T, Ferlitsch A, Sieghart W, Kreil A, Breitenecker F, Rieger A, Schmied B, Gangl A, Peck-Radosavljevic M (2010) HIV-HCV co-infected patients with low CD4+ cell nadirs are at risk for faster fibrosis progression and portal hypertension. J Viral Hepat 17(6):400–409
Thorpe J, Saeed S, Moodie EE, Klein MB; Canadian Co-infection Cohort Study (CTN222) (2011) Antiretroviral treatment interruption leads to progression of liver fibrosis in HIV-hepatitis C virus co-infection. AIDS 25(7):967–975
Shang D, Lin YH, Rigopoulou I, Chen B, Alexander GJM, Allain JP (2000) Detection of TT virus DNA in patients with liver disease and recipients of liver transplant. J Med Virol 61(4):455–461
Bendinelli M, Pistello M, Maggi F, Fornai C, Freer G, Vatteroni ML (2001) Molecular properties, biology, and clinical implications of TT virus, a recently identified widespread infectious agent of humans. Clin Microbiol Rev 14(1):98–113
Moriyama M, Matsumura H, Shimizu T, Shioda A, Kaneko M, Miyazawa K, Miyata H, Tanaka N, Uchida T, Arakawa Y (2001) Histopathologic impact of TT virus infection on the liver of type C chronic hepatitis and liver cirrhosis in Japan. J Med Virol 64(1):74–81
Sioda A, Moriyama M, Matsumura H, Kaneko M, Tanaka N, Arakawa Y (2001) Clinicopathological features of serum TTV DNA-positive non-A-G liver diseases in Japan. Hepatol Res 21(2):169–180
Masia G, Ingianni A, Demelia L, Faa G, Manconi PE, Pilleri G, Ciancio A, Rizzetto M, Coppola RC (2001) TT virus infection in Italy: prevalence and genotypes in healthy subjects, viral liver diseases and asymptomatic infections by parenterally transmitted viruses. J Viral Hepat 8(5):384–390
Shieh B, Chang MJ, Ko WC, Chen EJ, Wu JC, Lee CF, Chang TT, Li C (2003) Effects of multiple virus coinfections on disease progression in HIV-positive patients. Intervirology 46(2):105–113
Hofer H, Aydin I, Neumueller-Guber S, Mueller C, Scherzer TM, Staufer K, Steindl-Munda P, Wrba F, Ferenci P (2011) Prevalence and clinical significance of GB virus type C/hepatitis G virus coinfection in patients with chronic hepatitis C undergoing antiviral therapy. J Viral Hepat 18(7):513–517
Moriyama M, Matsumura H, Shimizu T, Shioda A, Kaneko M, Saito H, Miyazawa K, Tanaka N, Sugitani M, Komiyama K, Arakawa Y (2000) Hepatitis G virus coinfection influences the liver histology of patients with chronic hepatitis C. Liver 20(5):397–404
Acknowledgments
Additional contributions
We acknowledge the patients in this study for their participation and the Centro de Transfusión of Comunidad de Madrid for the healthy donor blood samples provided.
Potential conflicts of interest
None for all authors.
Funding/support
This work has been supported by grants from the “Instituto de Salud Carlos III (ISC-III)” (PI08/0738 and PI11/00245) to S.R.; from ISC-III (ref. ISCIII-RETIC RD06/006, PI08/0928) and the “Fundación para la Investigación y la Prevención del Sida en España” (FIPSE) (ref. 36443/03; ref. 361020/10) to J.B.; from ISC-III (INTRASALUD; RD09/0076/00103), Red RIS RD06-0006-0035, FIPSE (240800/09, 300509), and Fundación Caja Navarra and Comunidad de Madrid (S-SAL-0159-2006) to M.A.M.F. M.G.-F. and M.G.-A. are supported by a grant of Instituto de Salud Carlos III (CM09/00031 and CM08/00101, respectively). J.B. is supported by a grant from the “Programa de Intensificación de la Actividad Investigadora en el SNS” (I3SNS).
Writing assistance: Dr. Nick Weber provided writing assistance for this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
García-Álvarez, M., Berenguer, J., Álvarez, E. et al. Association of torque teno virus (TTV) and torque teno mini virus (TTMV) with liver disease among patients coinfected with human immunodeficiency virus and hepatitis C virus. Eur J Clin Microbiol Infect Dis 32, 289–297 (2013). https://doi.org/10.1007/s10096-012-1744-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-012-1744-1