Abstract
Purpose
To explore efficacy of the “Rey-Osterrieth complex figure (ROCF) tracing task” as a new test to detect unilateral spatial neglect (USN).
Methods
Subjects were 40 healthy control (HC) and 20 right brain-damaged patients with (USN + , n = 10) or without USN (USN − , n = 10). After the ROCF copying task, the tracing task was performed under conditions that did not leave any tracing lines on the sample figure. Evaluation used the conventional 36-point scoring system, laterality index (LI) as the ratio of the left and right structure scores, and the number of overlaps for each of the left and right structures scored.
Results
In the tracing task, USN + showed a lower LI than HC. Furthermore, left-sided neglect was sometimes more evident than in the copying task. Regarding the total overlapping score, USN + showed a greater score than HC. The right-sided overlapping scores in USN + and USN − were also greater than that in HC. In the right brain-damaged subjects, clinically meaningful correlations were not found between evaluations in the ROCF tracing task and in conventional USN screening tests. Receiver-operating-characteristic analysis to test the power of detection showed moderate performance for the tracing LI (AUC = 0.76, 95% CI = 0.54–0.97), which was greater than that of other tests. Further, the total overlapping score in the tracing task showed sensitivity 0.9 (highest among the tests performed), specificity 0.5, and AUC 0.68 (95% CI = 0.43–0.92).
Conclusion
The ROCF tracing task might be a convenient method to detect USN and to reveal the extent of spatial working memory impairment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Unilateral spatial neglect (USN) is defined as an impairment in reporting, responding to, or localizing stimuli presented to the contralateral side of a brain lesion [1], often occurring as left USN due to right-side brain damage [2]. USN manifests clinically as unilateral spatial inattention and trunk tilt, and it indicates poor prognosis due to an increased risk of falls [3], reduced activities of daily living (ADL) [4], and worsened quality of life [5], as well as an increased need for care after discharge [6] compared to patients without USN.
USN encompasses various subtypes, including egocentric and allocentric neglect [7], body space to distal space [8], and those related to visual search and time-related responses [9], which are assumed to be related to the site of injury [10] and affected by different mechanisms of onset [11, 12]. In a systematic review of the incidence of USN after stroke, Esposito et al. [13] reported that USN is seen in 45% of patients with right-side brain damage in the acute phase and in 20% of those in the chronic phase, but the prevalence of USN varies among studies. It has been pointed out that USN occurrence after stroke may be underestimated [14]. It is therefore recommended that a combination of cancellation, bisection, and copying tests be assessed, corresponding respectively to egocentric, allocentric, and egocentric/allocentric neglect [10, 15]. On the other hand, deficits in spatial working memory from posterior parietal cortex damage have been hypothesized to be involved in existence and severity of USN through the observation of ‘productive manifestations’ or “revisiting behavior” [16, 17]. In clinical practice, a simple and reliable testing method is needed to determine USN subtypes and guide clinicians to provide effective treatment according to the subtype. However, few methods have been reported for detecting USN in light of deficits in spatial working memory [16, 17, for the review see 10]. Wojciulk et al. [18] reported a new cancellation task that detects a behavior of repeated cancellation of items aggravated by the absence of visible cancellation marks.
We previously noted that some patients with right-side brain damage exhibit more USN symptoms in the tracing task—tracing without leaving tracing lines directly on the sample figure— than in the traditional copying task, as well as characteristic symptoms of repeatedly tracing the same lines over and over again. According to the phenomenon observed in the tracing task, patients with USN might not be able to remember where they have already traced and where they have not yet traced. On the other hand, the Rey-Osterrieth complex figure test (ROCF) [19, 20] is a neuropsychological assessment that is widely used as a copying or memorization task for which quantitative evaluation methods have been established. Therefore, we conceived the idea that ROCF could be used to quantify the characteristic phenomena that occur in the tracing task.
In the present pilot study, we propose “ROCF tracing task” as a new convenient method to detect USN and explore the method’s potential efficacies by assessing its correlation with conventional USN screening tests and its diagnostic utility.
Methods
This cross-sectional pilot study was designed to examine the characteristics of the tracing task and its potential use in clinical situations. To assess whether the “ROCF tracing task” can be used to identify USN, patients with right brain damage were divided into two groups according to presence or absence of USN based on screening tests. A group of healthy subjects was also included. To examine characteristics of the ROCF tracing task, the tracing task and a copy task [21] were performed using ROCF (i.e. the “ROCF copy task”) and scores were compared among the three groups. In addition, correlations between scores on the tracing task and scores on conventional USN screening methods (the cancellation test, line bisection test, and ROCF copy task) were computed. Finally, to characterize performance of the ROCF tracing task in terms of sensitivity and specificity, a receiver operating characteristic (ROC) curve was computed for each test in the two groups of patients with right brain damage.
Subjects
Subjects with right brain damage were recruited from patients admitted to Kirishima Rehabilitation Center of Kagoshima University Hospital. Healthy subjects were recruited from the general public.
The number of patients was set at 20 on the basis of cross-sectional studies of patients with right brain damage [22, 23].
The sample size of the healthy subject group was set at 40, double the number of right brain-damaged subjects, on the basis of cross-sectional studies of healthy controls and patients with right-brain damage [24, 25]. Thus, the patient and control sample sizes were different.
Inclusion and exclusion criteria
Inclusion criteria for patients with right brain damage were that they had had a first stroke, were right-handed, and were aged 20–80 years. Right brain damage was determined by consulting the medical history and by using medical information from the hospital where acute treatment was carried out, as well as by computed tomography images of the brain. Time since stroke onset was not considered in the patient inclusion criteria. Inclusion criterion for healthy subjects was being of age 20–80 years.
Exclusion criteria for both patients with right-brain damage and healthy subjects were a history of treatment for brain disorders (in the case of patients with right-brain-damage, a history of illness like stroke, brain injury, or surgery to the brain prior to the current stroke), mental illness or dementia which could affect the assessment results, or inability to understand the test description due to the effects of higher brain dysfunction or other factors.
We administered to participants various tasks described below, after obtaining written informed consent. This study complied with the Declaration of Helsinki and was approved by the Clinical Research Ethics Committee of Kagoshima University Hospital, Faculty of Medicine and Dentistry (No: 24–140).
USN screening
Presence of USN was determined by impaired performance on the USN screening test. The USN screening test consists of the line cancellation test [26], star cancellation test [27], line bisection test [27], and copying a landscape constructed of five objects [15] this is the method of assessment used in our hospital [28]. Cutoff values for the line bisection test and star cancellation test were based on the Japanese version of the Behavioral Inattention Test. The line cancellation test was judged by two or more omissions on the left half of the sheet compared with the number of omissions on the right half. The copying test was judged by omission of at least one detail of the left part. Patients with right-brain damage who showed symptoms of USN in any of the tests were assigned to the “USN + group”; other patients with right-brain damage were assigned to the “USN − group”.
Test and evaluations for the ROCF tracing and copying tasks
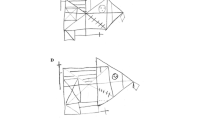
The ROCF tracing task was conducted immediately after the ROCF copying task. In the tracing task, subjects were presented with the ROCF sample and a sheet of carbon paper overlaid on a sheet of white paper, and instructed to trace the ROCF with chopsticks so as not to leave any tracing lines directly on the sample figure (Fig. 1a). At the same time, they were instructed not to trace the same spot more than once.
Both the tracing and copying tasks were evaluated conventionally with a maximum score of 36 points across 18 scoring units [29]. In addition, to identify differences between left and right structure copying and tracing, the left and right units were scored separately (Fig. 1b) (the left and right maximum scores were 12 and 16 points, respectively). In addition, the left–right ratio of correct responses was calculated as the Laterality Index (LI):
For example, an LI below 1 indicates a greater proportion of correct responses in the right structures, and an LI above 1 indicates a greater proportion of correct responses in the left structures.
Further, to score the frequencies of overlapping (i.e., tracing the same item) in the respective copying and tracing tasks, each scoring unit of the ROCF structures was scored 1 point for a complete overlap and 0.5 points for an incomplete overlap, and was calculated as the total, left, and right “overlapping scores” by adding the number of times the unit was traced with an overlap.
Procedure
Subjects were first interviewed about their education history and medical history related to the exclusion criteria, and tested using the Mini Mental State Examination-Japanese (MMSE-J) [30, 31]. The ROCF copying task and tracing task were then administered, on the same day.
The rules of the ROCF tracing task were explained to the subjects immediately after they had performed the ROCF copying task, and then they carried out the ROCF tracing task. If it was deemed that a subject did not understand the rules of the tracing task, the subject was asked to stop the task immediately and restart it after the rules were explained again. A subject was dismissed after having completed all of the tasks, and we then scored each task.
Statistical analyses
We compared right and left scores, LIs, and overlapping scores on the ROCF copying and tracing tasks among the three groups: healthy, USN − , and USN + . Primary outcome was the LIs on the ROCF tracing task, and the secondary outcome was the total overlapping score on the ROCF tracing task. The others were exploratory outcomes. The Shapiro–Wilk test was used to assess whether variables approximately followed a normal distribution; if lack of normality was judged, non-parametric tests (Kruskal–Wallis ANOVA followed by the Mann–Whitney U test with the Holm correction) were employed. A p-value of < 0.05 was considered to be statistically significant for the primary outcome. The effect size (r) was also calculated as r = √(t2/(t2 + df)).
To characterize the ROCF tracing task compared with the conventional USN screening test for subjects with right brain damage, we evaluated the correlations between scores or LI in the ROCF tracing task and those in the star cancellation test, line bisection test, or ROCF copying task, using Spearman's rank correlation coefficient. Furthermore, we performed Receiver Operating Characteristic (ROC) curve analysis to explore the performance, via the area under the ROC curve (AUC), of the scores and LI both in the ROCF tracing task as a USN detection test and in the conventional USN screening tests (i.e. the star cancellation test, line bisection test and the ROCF copying task). Tests with AUC greater than 0.9 were interpreted as highly accurate, 0.7–0.9 as moderately accurate, and 0.5–0.7 as rather inaccurate; an AUC of 0.5 represents a chance result [32].
All statistical analyses were performed using R (The R Foundation for Statistical Computing, version 4. 0. 2).
Results
The examinations were carried out with 40 healthy volunteers and 20 patients with right-brain damage: 10 patients were in the USN + group and the other 10 were in the USN − group. The characteristics of each group are presented in Table 1. There were no extreme differences in age, gender, school attendance, or MMSE-J scores among the three groups, nor were there any between the USN + and USN − groups in duration from onset or proportion of cerebral infarction and hemorrhage diagnoses.
Figure 2 shows an example of the performance by a patient with USN in the ROCF copying task (Fig. 2a) and the tracing task (Fig. 2b). He obtained a total of 32 points and an LI of 0.83 in the copying task, and a total of 24 points and an LI of 0.72 in the tracing task. In case of overlapping scores, while he obtained 0 points in total for the copying task, the right score was 1.5 points, the left score was 1 point, and the total score was 2.5 points for the tracing task.
ROCF copying task scores and LI
The ROCF copying scores and LI in the healthy, USN − , and USN + groups are shown in Table 2. Both the USN + group and the USN − group had lower copying scores than the healthy controls, but little difference were found between the USN + and USN − groups. On the other hand, USN + had an LI that was smaller than that in the healthy controls; little differences in LI were found between the USN + and USN − groups.
ROCF tracing task scores and LI
Table 3 shows the summary of the ROCF tracing scores and LI in the 3 groups. While there were no significant differences among the 3 groups in the tracing scores, a significant between-group difference was seen in LI: while the USN − group did not differ significantly from the healthy controls, the USN + group showed a smaller LI than the controls (p < 0.001, r = 0.42). The median LI for the USN + group was 0.84 (interquartile range [IQR] 0.75–0.90) and for the USN– group was 0.86 (IQR 0.86–0.97), and subjects in the USN + group lost more points on the left side of the ROCF tracing task than subjects in the USN– group. Clinically, in a few patients with or without USN, left-sided neglect was more pronounced in the tracing than in the copying task.
Overlapping scores for the ROCF copying task and tracing task
The summary of the overlapping scores for the copying task and tracing task in the 3 groups are shown in Table 4. In the ROCF copying task, there were no significant differences in the right, left, or total overlapping scores among the 3 groups. In the ROCF tracing task, however, the right, left, and total overlapping scores evidenced possible significant differences among the 3 groups. In post-hoc pairwise comparisons, the right overlapping score was greater in the USN + and USN − groups than in the controls. On the contrary, the left overlapping score in the USN + group was greater than in the controls, but not that in the USN − group. The left overlapping score in the USN − group was slightly greater than in the controls. For the total overlapping score, only the USN + group showed a significantly greater score than in the controls.
Correlation and diagnostic utility of the ROCF tracing task with conventional USN screening tests
Correlations between the ROCF tracing task and the conventional USN screening tests are shown in Table 5. Clinically meaningful correlations were not found between the ROCF tracing task and conventional USN screening tests.
A summary of performance analysis based on ROC curves for evaluations in the ROCF tracing task and conventional USN detection task is shown in Table 6. Specificity and sensitivity of each task for a chosen threshold (cutoff point) are shown along with values of AUC for ROC curves with varying cutoff points. Cutoff points for each task were chosen by the point on the ROC curve closest to the upper left-hand corner (100% sensitivity and 100% specificity) [32]. Confidence intervals for AUC were computed by DeLong’s method [33]. In the conventional USN detection tasks (such as the cancellation test, line bisection test, and the copying score), sensitivities were 0.5 or less, although they showed relatively high specificity of 0.7 or more. In contrast, in the ROCF tracing task, tracing LI showed sensitivity 0.8, specificity 0.7; AUC was 0.76, implying that is moderately accurate (95% CI = 0.54–0.97), and had the highest AUC (best performance) among the tests performed. Further, total overlapping score showed 70% beyond the cutoff point, the sensitivity 0.9, specificity 0.5; AUC 0.68 (95% CI = 0.43–0.92), and had the highest sensitivity among the tests performed.
Discussion
In this study, we compared the performance of the copying task and the tracing task using the ROCF in three groups: a healthy control group, a USN − group (absence of USN based on USN screening after right-side brain damage), and a USN + group (presence of USN after right-side brain damage).
ROCF copying scores among the healthy group, the USN − group, and the USN + group differed in clinical meaningful ways; however, differences in ROCF tracing scores among the three groups were not clinically meaningful. In addition, both the USN + and USN − groups (i.e., patients with right-side brain damage) showed lower copying scores than did the healthy controls, but not lower tracing scores. Four main cognitive processes have been postulated to be active in figure copying: visual and spatial analysis, drawing plan preparation, execution, and control processes [34]; previous studies comparing brain activity in copying and tracing in healthy subjects have reported stronger activation in the bilateral interparietal sulci, premotor cortex, and supplementary motor cortex in copying [35]. Constructional apraxia often occurs in patients with right-side brain damage and impairs their ability to discern precise spatial relationships between the components of an object [36], making it difficult to copy drawings. It has been reported that tracing is possible even in the presence of constructional apraxia [37], so the ROCF tracing task was expected to be easier than the copying task. Thus, the USN + and USN − groups could have ROCF tracing scores comparable to those of healthy controls, but not so for copying scores. In total, however, we should consider several factors that influence the difficulties of the copying and tracing scores: (1) the copying task was carried out immediately before the tracing task; (2) the participants were instructed not to trace the same lines repeatedly; and (3) the task condition of the present tracing, which did not leave tracing lines after tracing, increased the burden on the motor working memory.
In the ROCF tracing task, the LI in the USN + group was significantly smaller than that in the control group, and was somewhat smaller than that in the USN − group. Leyland et al. [38] reported that when patients with USN were presented with two portraits in a row and asked to copy and trace them, they showed allocentric neglect symptoms in copying and egocentric neglect symptoms in tracing. The present results with the ROCF tracing task suggest that the nature of the ROCF, in which each unit is scored according to its shape and position, reduced the number of errors due to constructional apraxia after right-side brain damage, and also revealed omissions of the left construction due to the induction of an egocentric USN. This suggests that, although it is difficult to determine the presence or degree of USN in patients with right-sided brain injury based on their scores in the ROCF tracing task, calculating LI might more clearly reveal the presence or absence of USN, particularly the egocentric aspect of USN, which has traditionally been determined by cancellation tests or copying tasks [39].
In the ROCF copying task, there were few overlapping in all the three groups. However, in the tracing task, more overlapping on the right side was observed in the USN– group than in the healthy group, and in the USN + group than in the USN– group. Wojciulik et al. [17, 18] conducted a cancellation test in patients with right–brain damage under conditions that did not leave a trace, and they found a stronger degree of USN with that test than with the conventional cancellation task with a trace, as well as the repeated canceling of items on the right side that had already been canceled; they suggested that this was due to a spatial working memory deficit. In addition, Malhotra et al. [40] reported that poor performance in spatial working memory tasks correlated with the severity of USN in cancellation tasks. Moreover, Wansard et al. [41] reported that a group of patients with USN in a computerized task using a touch screen exhibited impaired spatial working memory and many re-cancellations. In addition to deficits in spatial working memory, the involvement of spatial remapping deficits has also been reported as a cause of such phenomena occurring in USN [42]. Many previous studies examined cancellation tasks without leaving tracing lines, and in our ROCF tracing task, we used a single figure that did not require exploration, so it seems unlikely that spatial remapping deficits would be affected, and overlapping of the left and right structures is thought to be caused by a deficit in spatial working memory. In addition, the USN + group showed a significantly greater overlapping score for the left side than in the control group, and the left score in the USN − group was slightly greater than that in the controls. Rode et al. proposed the term “hyperschematia” for the excessive writing and leftward expansion in left space in drawing and copying in right brain-damaged patients, and considered that a leftward relaxation of the spatial medium might be involved and might not be related to the USN [43]. Therefore, in addition to deficits in the spatial working memory, certain aspects of “hyperschematia” might have influenced the present results of greater overlapping scores on the left side of patients with right-brain damage.
Conventional USN screening tests and tracing tasks in patients with right-brain damage showed no clinically significant correlations. This suggests that the ROCF tracing task may reveal USN symptoms that cannot be revealed in the copying task. The fact that overlapping scores in ROCF tracing task did not show clinically meaningful correlations with the other tests may be because the overlapping scores might focus on the spatial working memory, which was not tested in the conventional USN screening test.
ROC curve analysis of the ROCF tracing task and conventional USN screening test showed moderately accurate AUC only for the ROCF tracing LI and the highest sensitivity for the tracing total overlapping score. Conventional USN screening tests have been noted to have low sensitivity and high specificity [44], and this was also true for the results of conventional screening tests in this study. The ROCF tracing LI and overlapping score, which recognized high sensitivity and AUC, were expected to be effective in reducing missed USN when combined with conventional screening tests.
These results suggest that ROCF tracing LI produced a load on spatial working memory and had power to detect egocentric USN that was not evident in the line cancellation test, the star cancellation test, or the copying task, and that the total overlapping score could detect USN with high sensitivity by assessing spatial working memory impairment. It was also suggested that the left overlapping score may reveal hyperschematia in patients with USN, which was previously thought to be non-comorbid [43].
In addition, this study used tasks with paper and pencil (and chopsticks) that were used in everyday clinical situations, which was considered a strength as it can be easily used to detect USN with greater sensitivity than conventional tests, and assess spatial working memory in USN in any clinical situation simply and conveniently.
A limitation of this study is that, as it was a cross-sectional study, the timing of onset in patients with right-side brain damage varied from subacute to chronic and it is not possible to determine how findings in the tracing task would change with improvements in USN. In the future, the mechanistic aspects of USN in the ROCF tracing task should be clarified in more detail by examining correlations between changes over time in the ROCF tracing task as well as other USN assessment tests and ADL. In addition, this study did not examine associations between brain lesions and scores in the ROCF tracing task. Future studies analyzing these associations might allow verification of what the ROCF tracing task can reveal about the mechanism of USN. Another limitation of the study is that, due to the small sample size, cutoff values for each score of the ROCF tracing task could not be established with precision.
In summary, the ROCF-based tracing task has the potential to reveal the presence of USN and the impairment of spatial working memory for existing copying tasks. In conclusion, the ROCF tracing task could be a convenient and highly detectable novel method for assessing USN.
Data availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
References
Heilman KM, Watson RT, Valenstein E (2002) Spatial neglect. In: Karnath H-O, Milner D, Vallar G (eds) The cognitive and neural bases of spatial neglect .Oxford University Press, New York, pp 3–30. https://doi.org/10.1093/acprof:oso/9780198508335.003.0001
Ringman JM, Saver JL, Woolson RF et al (2004) Frequency, risk factors, anatomy, and course of unilateral neglect in an acute stroke cohort. Neurology 63:468–474. https://doi.org/10.1212/01.wnl.0000133011.10689.ce
Chen P, Hreha K, Kong Y, Barrett AM (2015) Impact of spatial neglect on stroke rehabilitation: evidence from the setting of an inpatient rehabilitation facility. Arch Phys Med Rehabil 96:1458–1466. https://doi.org/10.1016/j.apmr.2015.03.019
Spaccavento S, Cellamare F, Falcone R et al (2017) Effect of subtypes of neglect on functional outcome in stroke patients. Ann Phys Rehabil Med 60:376–381. https://doi.org/10.1016/j.rehab.2017.07.245
Sobrinho KRF, Santini ACM, Marques CLS et al (2018) Impact of unilateral spatial neglect on chronic patient’s post-stroke quality of life. Somatosens Mot Res 35:199–203. https://doi.org/10.1080/08990220.2018.1521791
Barrett AM (2021) Spatial Neglect and Anosognosia After Right Brain Stroke. Continuum (Minneap Minn) 27:1624–1645. https://doi.org/10.1212/CON.0000000000001076
Grimsen C, Hildebrandt H, Fahle M (2008) Dissociation of egocentric and allocentric coding of space in visual search after right middle cerebral artery stroke. Neuropsychologia 46:902–914. https://doi.org/10.1016/j.neuropsychologia.2007.11.028
Committeri G, Pitzalis S, Galati G et al (2007) Neural bases of personal and extrapersonal neglect in humans. Brain 130:431–441. https://doi.org/10.1093/brain/awl265
Becchio C, Bertone C (2006) Time and neglect: abnormal temporal dynamics in unilateral spatial neglect. Neuropsychologia 44:2775–2782. https://doi.org/10.1016/j.neuropsychologia.2006.06.011
Rode G, Pagliari C, Huchon L et al (2017) Semiology of neglect: An update. Ann Phys Rehabil Med 60:177–185. https://doi.org/10.1016/j.rehab.2016.03.003
Coulthard E, Parton A, Husain M (2006) Action control in visual neglect. Neuropsychologia 44:2717–2733. https://doi.org/10.1016/j.neuropsychologia.2005.11.004
Malhotra P, Coulthard E, Husain M (2006) Hemispatial neglect, balance and eye-movement control. Curr Opin Neurol 19:14–20. https://doi.org/10.1097/01.wco.0000198101.87670.7e
Esposito E, Shekhtman G, Chen P (2021) Prevalence of spatial neglect post-stroke: A systematic review. Ann Phys Rehabil Med 64:101459. https://doi.org/10.1016/j.rehab.2020.10.010
Puig-Pijoan A, Giralt-Steinhauer E, Zabalza de Torres A et al (2018) Underdiagnosis of Unilateral Spatial Neglect in stroke unit. Acta Neurol Scand 138:441–446. https://doi.org/10.1111/ane.12998
Moore M, Milosevich E, Beisteiner R et al (2022) Rapid screening for neglect following stroke: A systematic search and European Academy of Neurology recommendations. Eur J Neurol 29:2596–2606. https://doi.org/10.1111/ene.15381
Husain M, Mannan S, Hodgson T et al (2001) Impaired spatial working memory across saccades contributes to abnormal search in parietal neglect. Brain 124:941–952. https://doi.org/10.1093/brain/124.5.941
Wojciulik E, Husain M, Clarke K, Driver J (2001) Spatial working memory deficit in unilateral neglect. Neuropsychologia 39:390–396. https://doi.org/10.1016/s0028-3932(00)00131-7
Wojciulik E, Rorden C, Clarke K et al (2004) Group study of an “undercover” test for visuospatial neglect: invisible cancellation can reveal more neglect than standard cancellation. J Neurol Neurosurg Psychiatry 75:1356–1358. https://doi.org/10.1136/jnnp.2003.021931
Rey A (1942) L’examen psychologique dans les cas d’encephalopathie traumatic [Psychological assessment of brain trauma]. Arch Psychol 28:286–340
Osterrieth PA (1944) Le test de copie d’une figure complexe; contribution a l’etude de la perception et de la memoire [Test of copying a complex figure; contribution to the study of perception and memory]. Arch Psychol 30:206–356
Gainotti G, D’Erme P, Monteleone D, Silveri MC (1986) Mechanisms of unilateral spatial neglect in relation to laterality of cerebral lesions. Brain 109(Pt 4):599–612. https://doi.org/10.1093/brain/109.4.599
Marques CLS, de Souza JT, Gonçalves MG et al (2019) Validation of the Catherine Bergego Scale in patients with unilateral spatial neglect after stroke. Dement Neuropsychol 13:82–88. https://doi.org/10.1590/1980-57642018dn13-010009
de Sire A, Baricich A, Ferrillo M, Migliario M, Cisari C, Invernizzi M (2020) Buccal hemineglect: is it useful to evaluate the differences between the two halves of the oral cavity for the multidisciplinary rehabilitative management of right brain stroke survivors? A cross-sectional study. Top Stroke Rehabil 27:208–214. https://doi.org/10.1080/10749357.2019.1673592
Narison R, De Montalembert M, Conty L (2020) Diagnosing gaze and arrow cueing effects in unilateral spatial neglect. Neurocase 26:42–50. https://doi.org/10.1080/13554794.2019.1705495
Narison R, de Montalembert M, Bayliss A, Conty L (2021) Measuring Gaze and Arrow Cuing Effects With a Short Test Adapted to Brain Damaged Patients With Unilateral Spatial Neglect: A Preliminary Study. Front Psychol 29(12):690197. https://doi.org/10.3389/fpsyg.2021.690197
Albert ML (1973) A simple test of visual neglect. Neurology 23:658–664. https://doi.org/10.1212/wnl.23.6.658
Wilson B, Cockburn J, Halligan P (1987) Behavioural inattention test. Thames Valley Test Company, Suffolk, England
Shimodozono M, Matsumoto S, Miyata R et al (2006) Perceptual, premotor and motor factors in the performance of a delayed-reaching task by subjects with unilateral spatial neglect. Neuropsychologia 44:1752–1764. https://doi.org/10.1016/j.neuropsychologia.2006.03.012
Taylor LB (1998) Scoring criteria for the Rey-Osterrieth Complex Figure Test. In: Spreen O, Strauss E (eds) A compendium of neuropsychological tests Administration, norms, and commentary pp 810–837. Oxford University Press, Oxford
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198. https://doi.org/10.1016/0022-3956(75)90026-6
Sugishita M (2012) Mini mental state examination-Japanese. Nihon Bunka Kagakusya Company, Tokyo
Akobeng AK (2007) Understanding diagnostic tests 3: receiver operating characteristic curves. Acta Paediatr 96:644–647. https://doi.org/10.1111/j.1651-2227.2006.00178.x
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
Trojano L, Grossi D (1998) “Pure” constructional apraxia - a cognitive analysis of a single case. Behav Neurol 11:43–49. https://doi.org/10.1155/1998/614728
Ogawa K, Inui T (2009) The role of the posterior parietal cortex in drawing by copying. Neuropsychologia 47:1013–1022. https://doi.org/10.1016/j.neuropsychologia.2008.10.022
Russell C, Deidda C, Malhotra P et al (2010) A deficit of spatial remapping in constructional apraxia after right-hemisphere stroke. Brain 133:1239–1251. https://doi.org/10.1093/brain/awq052
Gainotti G, Trojano L (2018) Constructional apraxia. In: Vallar G, Coslett HB (eds) Handbook of Clinical Neurology. Elsevier, Amsterdam, pp 331–348
Leyland L-A, Godwin HJ, Benson V, Liversedge SP (2017) Neglect Patients Exhibit Egocentric or Allocentric Neglect for the Same Stimulus Contingent upon Task Demands. Sci Rep 7:1941. https://doi.org/10.1038/s41598-017-02047-x
Chechlacz M, Rotshtein P, Humphreys GW (2012) Neuroanatomical Dissections of Unilateral Visual Neglect Symptoms: ALE Meta-Analysis of Lesion-Symptom Mapping. Front Hum Neurosci 6:230. https://doi.org/10.3389/fnhum.2012.00230
Malhotra P, Jäger HR, Parton A et al (2005) Spatial working memory capacity in unilateral neglect. Brain 128:424–435. https://doi.org/10.1093/brain/awh372
Wansard M, Meulemans T, Gillet S et al (2014) Visual neglect: is there a relationship between impaired spatial working memory and re-cancellation? Exp Brain Res 232:3333–3343. https://doi.org/10.1007/s00221-014-4028-4
Pisella L, Mattingley JB (2004) The contribution of spatial remapping impairments to unilateral visual neglect. Neurosci Biobehav Rev 28:181–200. https://doi.org/10.1016/j.neubiorev.2004.03.003
Rode G, Ronchi R, Revol P, et al (2014) Hyperschematia after right brain damage: a meaningful entity? Front Hum Neurosci 8:. https://doi.org/10.3389/fnhum.2014.00008
Grech M, Stuart T, Williams L et al (2017) The Mobility Assessment Course for the Diagnosis of Spatial Neglect: Taking a Step Forward? Front Neurol 8:563. https://doi.org/10.3389/fneur.2017.00563
Funding
Open access funding provided by Kagoshima University. Partial financial support was received from Tarumizu Chuo Hospital.
Author information
Authors and Affiliations
Contributions
RO: study concept and design, analysis of data and interpretation, manuscript writing.
SM: study design, analysis of data and interpretation, manuscript reviewing.
YO: acquisition of data, critical revision of the manuscript for important intellectual content.
KY: acquisition of data, interpretation of data.
MS: study concept and design, data interpretation, critical revision of the manuscript for important intellectual content, study supervision.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Clinical Research Ethics Committee of Kagoshima University Hospital, Faculty of Medicine and Dentistry (No: 24–140).
Informed Consent
Written informed consent was obtained from all subjects included.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ohama, R., Matsumoto, S., Ohama, Y. et al. A new method for detecting unilateral spatial neglect with tracing tasks using the Rey-Osterrieth complex figure: a pilot study. Neurol Sci (2024). https://doi.org/10.1007/s10072-024-07540-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10072-024-07540-6