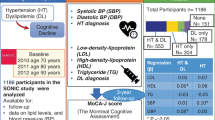
Abstract
Background
Hypertension is an established risk factor for mild cognitive impairment (MCI) in elderly individuals. Nevertheless, the impact of different levels of blood pressure on the progression of MCI remains uncertain. This study aims to investigate the non-linear relationship between blood pressure and MCI in the elderly and detect the critical blood pressure threshold, thus, improving blood pressure management for individuals at high risk of MCI.
Methods
Data was obtained from the Chinese Longitudinal Healthy Longevity Survey (CLHLS) cohort. We chose normal cognitive elderly individuals who entered the cohort in 2014 for a 5-year follow-up to observe the progression of MCI. Subsequently, we utilized the Cox regression model to identify risk factors for MCI and conducted a Cox-based restricted cubic spline regression (RCS) model to examine the non-linear relationship between systolic blood pressure (SBP) and diastolic blood pressure (DBP) with MCI, determining the critical blood pressure threshold for MCI progression.
Results
In the elderly population, female (HR = 1.489, 95% CI: 1.017–2.180), lacking of exercise in the past (HR = 1.714, 95% CI: 1.108–2.653), preferring animal fats (HR = 2.340, 95% CI: 1.348–4.061), increased age (HR = 1.061, 95% CI: 1.038–1.084), increased SBP (HR = 1.036, 95% CI: 1.024–1.048), and increased DBP (HR = 1.056, 95% CI: 1.031–1.081) were associated with MCI progression. After adjusting factors such as gender, exercise, preferred types of fats, and age, both SBP (P non-linear < 0.001) and DBP (P non-linear < 0.001) in elderly individuals exhibited a non-linear association with MCI. The risk of MCI rose when SBP exceeded 135 mmHg and DBP was in the range of 80–88 mmHg. However, when DBP exceeded 88 mmHg, there was a declining trend in MCI progression, although the HR remained above 1. The identified critical blood pressure management threshold for MCI was 135/80 mmHg.
Conclusion
In this study, we discovered that risk factors affecting the progression of MCI in elderly individuals comprise gender (female), preferring to use animal fat, lack of exercise in the past, increased age, increased SBP, and increased DBP. Additionally, a non-linear relationship between blood pressure levels and MCI progression was confirmed, with the critical blood pressure management threshold for MCI onset falling within the prehypertensive range.






Similar content being viewed by others
Data availability
Any data not published within the article is available in a public repository.
References
Li X, Zhang ZJ (2015) Neuropsychological and neuroimaging characteristics of amnestic mild cognitive impairment subtypes: a selective overview. CNS Neurosci Ther 21:776–783
Qiu S, Joshi PS, Miller MI et al (2020) Development and validation of an interpretable deep learning framework for Alzheimer’s disease classification. Brain 143:1920–1933. https://doi.org/10.1093/brain/awaa137
Nagaraj S, Duong TQ (2021) Deep Learning and Risk Score Classification of Mild Cognitive Impairment and Alzheimer’s Disease. J Alzheimers Dis 80:1079–1090. https://doi.org/10.1101/2020.11.09.20226746
Ward DD, Wallace LM, Rockwood K (2021) Frailty and risk of dementia in mild cognitive impairment subtypes. Ann Neurol 89:1221–1225. https://doi.org/10.1002/ana.26064
McCollum L, Karlawish J (2020) Cognitive impairment evaluation and management. Medical Clinics 104:807–825. https://doi.org/10.1016/j.mcna.2020.06.007
Jin X, He W, Zhang Y et al (2021) Association of APOE ε4 genotype and lifestyle with cognitive function among Chinese adults aged 80 years and older: A cross-sectional study. PLoS Med 18:e1003597. https://doi.org/10.1371/journal.pmed.1003597
Santisteban MM, Iadecola C, Carnevale D (2023) Hypertension, neurovascular dysfunction, and cognitive impairment. Hypertension 80:22–34. https://doi.org/10.1161/HYPERTENSIONAHA.122.18085
Gorelick PB, Scuteri A, Black SE et al (2011) Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42:2672–2713. https://doi.org/10.1161/STR.0b013e3182299496
Biessels GJ, Despa F (2018) Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat Rev Endocrinol 14:591–604. https://doi.org/10.1038/s41574-018-0048-7
Zackova L, Jani M, Brazdil M et al (2021) Cognitive impairment and depression: meta-analysis of structural magnetic resonance imaging studies. NeuroImage: Clin 32:102830. https://doi.org/10.1016/j.nicl.2021.102830
Cordani C, Young VM, Arienti C et al (2022) Cognitive impairment, anxiety and depression: A map of Cochrane evidence relevant to rehabilitation for people with post COVID-19 condition. EuropEan J Phys Rehabil Med 58:880. https://doi.org/10.23736/s1973-9087.22.07813-3
McInnes K, Friesen CL, MacKenzie DE et al (2017) Mild Traumatic Brain Injury (mTBI) and chronic cognitive impairment: A scoping review. PLoS ONE 12:e0174847. https://doi.org/10.1371/journal.pone.0174847
Lövdén M, Fratiglioni L, Glymour MM et al (2020) Education and cognitive functioning across the life span. Psychol Sci Publ Interes 21:6–41. https://doi.org/10.1177/1529100620920576
Vanek J, Prasko J, Genzor S et al (2020) Obstructive sleep apnea, depression and cognitive impairment. Sleep Med 72:50–58. https://doi.org/10.1016/j.jagp.2016.01.134
Livingston G, Huntley J, Sommerlad A et al (2020) Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet 396:413–446. https://doi.org/10.1016/S0140-6736(20)30367-6
Si J-P, Jiang H, Li Y-Y (2020) Correlation between effectual time and the curative effect in patients with all frequency descending sudden deafness after treatment. Am J Otolaryngol 41:102621. https://doi.org/10.1016/j.amjoto.2020.102621
Qin J, He Z, Wu L et al (2021) Prevalence of mild cognitive impairment in patients with hypertension: a systematic review and meta-analysis. Hypertens Res 44:1251–1260. https://doi.org/10.1038/s41440-021-00704-3
Wang J, Peng G, Wang X (2023) Relationship between blood pressure variability and mild cognitive dysfunction in elderly patients with hypertension. Chin J Geriatr Heart Brain Vessel Dis 25:988–990
Heizhati M, Li N, Wang L et al (2021) Association of Hypertension with mild cognitive impairment in population from less-developed areas of multiethnic Northwest China. Neuroepidemiology 55:407–415. https://doi.org/10.1159/000517956
Tzourio C, Dufouil C, Ducimetière P et al (1999) Cognitive decline in individuals with high blood pressure: a longitudinal study in the elderly. Neurology 53:1948–1948. https://doi.org/10.1212/WNL.53.9.1948
Waldstein SR, Giggey PP, Thayer JF et al (2005) Nonlinear relations of blood pressure to cognitive function: the Baltimore Longitudinal Study of Aging. Hypertension 45:374–379. https://doi.org/10.1161/01.HYP.0000156744.44218.74
Thorvaldsson V, Skoog I, Hofer SM et al (2012) Nonlinear blood pressure effects on cognition in old age: separating between-person and within-person associations. Psychol Aging 27:375. https://doi.org/10.1037/a0025631
Zhang M, Lv X, Chen Y et al (2022) Excessive sleep increased the risk of incidence of cognitive impairment among older Chinese adults: a cohort study based on the Chinese Longitudinal Healthy Longevity Survey (CLHLS). Int Psychogeriatr 34:725–734. https://doi.org/10.1017/S1041610221000168
An R, Liu GG (2016) Cognitive impairment and mortality among the oldest-old Chinese. Int J Geriatr Psychiatry 31:1345–1353. https://doi.org/10.1002/gps.4442
Hu M, Shu X, Yu G et al (2021) A risk prediction model based on machine learning for cognitive impairment among Chinese community-dwelling elderly people with normal cognition: development and validation study. J Med Internet Res 23:e20298. https://doi.org/10.3389/fpubh.2023.1143019
Yu X, Zhang W, Kobayashi LC (2021) Duration of subjective poverty in relation to subsequent cognitive performance and decline among adults aged ≥64 in China, 2005–2018. Soc Sci Med 283:114188. https://doi.org/10.1016/j.socscimed.2021.114188
Feng T, Feng Z, Liu Q et al (2021) Drinking habits and water sources with the incidence of cognitive impairment in Chinese elderly population: The Chinese Longitudinal Healthy Longevity Survey. J Affect Disord 15:406–412. https://doi.org/10.1016/j.jad.2020.12.044
Bai W, Chen P, Cai H et al (2022) Worldwide prevalence of mild cognitive impairment among community dwellers aged 50 years and older: a meta-analysis and systematic review of epidemiology studies. Age Ageing 51:afa173. https://doi.org/10.1093/ageing/afac173
Yang J, Zhao X, Sui H et al (2021) High prevalence and low awareness of mild cognitive impairment in a suburban community in Shanghai. Neurol India 69:1693. https://doi.org/10.4103/0028-3886.333524
Song M, Wang Y-M, Wang R et al (2021) Prevalence and risks of mild cognitive impairment of Chinese community-dwelling women aged above 60 years: A cross-sectional study. Arch Women’s Mental Health 24:903–911. https://doi.org/10.1016/S2468-2667(20)30185-7
Xu S, Xie B, Song M et al (2014) High prevalence of mild cognitive impairment in the elderly: a community-based study in four cities of the Hebei Province, China. Neuroepidemiology 42:123–130. https://doi.org/10.1159/000357374
Overton M, Pihlsgård M, Elmståhl S (2019) Prevalence and incidence of mild cognitive impairment across subtypes, age, and sex. Dement Geriatr Cogn Disord 47:219–232
Sakurai K, Shen C, Shiraishi I et al (2021) Consumption of oleic acid on the preservation of cognitive functions in Japanese elderly individuals. Nutrients 13:284. https://doi.org/10.1159/000499763
Marti A, Fortique F (2019) Omega-3 fatty acids and cognitive decline: a systematic review. Nutr Hosp 36:939–949. https://doi.org/10.1159/000499763
Maltais M, Lorrain D, Leveille P et al (2022) Long-chain Omega-3 fatty acids supplementation and cognitive performance throughout adulthood: A 6-month randomized controlled trial. Prostaglandins Leukot Essent Fatty Acids 178:102415. https://doi.org/10.7205/MILMED-D-14-00168
Zhu R-z, Chen M-q, Zhang Z-w et al (2021) Dietary fatty acids and risk for Alzheimer’s disease, dementia, and mild cognitive impairment: a prospective cohort meta-analysis. Nutrition 90:111355. https://doi.org/10.1016/j.nut.2021.111355
Centers for Disease Control and Prevention (CDC) (2013) Self-reported increased confusion or memory loss and associated functional difficulties among adults aged≥ 60 years-21 states, 2011. Morb Mortal Wkly Rep 62:345–350
Langa KM, Levine DA (2014) The diagnosis and management of mild cognitive impairment: a clinical review. JAMA 312:2551–2561. https://doi.org/10.1001/jama.2014.13806
Anheyer D, Klose P, Koch AK et al (2020) Comparative efficacy of different exercise interventions in chronic non-specific low back pain: protocol of a systematic review and network meta-analysis. BMJ Open 10:e036050. https://doi.org/10.1136/bmjopen-2019-036050
Ungvari Z, Toth P, Tarantini S et al (2021) Hypertension-induced cognitive impairment: from pathophysiology to public health. Nat Rev Nephrol 17:639–654. https://doi.org/10.1038/s41581-021-00430-6
de Menezes ST, Giatti L, Brant LCC et al (2021) Hypertension, prehypertension, and hypertension control: association with decline in cognitive performance in the ELSA-Brasil cohort. Hypertension 77:672–681. https://doi.org/10.1161/HYPERTENSIONAHA.120.16080
Ou Y-N, Tan C-C, Shen X-N et al (2020) Blood pressure and risks of cognitive impairment and dementia: a systematic review and meta-analysis of 209 prospective studies. Hypertension 76:217–225. https://doi.org/10.1161/HYPERTENSIONAHA.120.14993
Acknowledgements
Data used in this study was derived from the Chinese Longitudinal Healthy Longevity Survey (CLHLS) cohort (https://opendata.pku.edu.cn/dataverse/CHADS). Launched in 1998, the CLHLS conducted follow-up surveys in 2000, 2002, 2005, 2008-2009, 2011-2012, 2014, and 2017-2018. This cohort spans the majority of the provinces in China and aims to investigate the factors associated with healthy human longevity. It offers a wealth of comprehensive information, including socio-demographic characteristics, health status, and daily activities of the elderly.
Funding
The study was supported by the Key Science and Technology Program Projects of Zigong, China (2022ZCNKY19).
Author information
Authors and Affiliations
Contributions
The paper was and written by FY. FY and YG were responsible for the overall idea design of the paper. XL, YY, QX, and YY reviewed and substantively revised the paper. QZ and CL were responsible for data acquisition and analysis, MN and QW responsible for quality evaluation of kinds of literature. YZ served as the overall lead and took responsibility for the paper. All authors listed made significant contributions to the work, both in terms of direct involvement and intellectual input.
Corresponding author
Ethics declarations
Ethical approval and Informed consent
The CLHLS cohort study has obtained approval from Peking University’s Research Ethics Committee (IRB00001052-13074). All participants or their authorized representatives have provided written informed consent.
Consent for publication
This manuscript has been approved for publication by all authors.
Conflict of interest
The authors have no conflict of interest to report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yi, F., Gao, Y., Liu, X. et al. A non-linear relationship between blood pressure and mild cognitive impairment in elderly individuals: A cohort study based on the Chinese longitudinal healthy longevity survey (CLHLS). Neurol Sci (2024). https://doi.org/10.1007/s10072-024-07539-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10072-024-07539-z