Abstract
Background
Verbal fluency (VF) tasks are known as suitable for detecting cognitive impairment (CI) in Parkinson’s disease (PD). This study thus aimed to evaluate the psychometrics and diagnostics of the Alternate Verbal Fluency Battery (AVFB) by Costa et al. (2014) in an Italian cohort of non-demented PD patients, as well as to derive disease-specific cut-offs for it.
Methods
N = 192 non-demented PD patients were screened with the Montreal Cognitive Assessment (MoCA) and underwent the AVFB—which includes phonemic, semantic and alternate VF tests (PVF; SVF; AVF), as well as a Composite Shifting Index (CSI) reflecting the “cost” of shifting from a single- to a double-cued VF task. Construct validity and diagnostics were assessed for each AVFB measure against the MoCA. Internal reliability and factorial validity were also tested.
Results
The MoCA proved to be strongly associated with PVF, SVF and AVF scores, whilst moderately with the CSI. The AVFB was internally consistent and underpinned by a single component; however, an improvement in both internal reliability and fit to its factorial structure was observed when dropping the CSI. Demographically adjusted scores on PVF, SVF and AVF tests were diagnostically sound in detecting MoCA-defined cognitive impairment, whilst this was not true for the CSI. Disease-specific cut-offs for PVF, SVF and AVF tests were derived.
Discussion
In conclusion, PVF, SVF and AVF tests are reliable, valid and diagnostically sound instruments to detect cognitive impairment in non-demented PD patients and are therefore recommended for use in clinical practice and research.
Similar content being viewed by others
Background
Up to 40% of non-demented patients with Parkinson’s disease (PD) present with dysexecutive-like, widespread cognitive impairment (CI) [1], which adversely affects their functional outcomes [2], prognosis [3, 4] and survival [5]. Therefore, the early detection of CI via clinimetrically sound tests is clinically crucial in this population [6].
Verbal fluency (VF) tests have been systematically found to be appropriate for this goal [7], as they capture both dysexecutive-inattentive features and lexical-semantic deficits that characterize PD [8] also in the early stages [9,10,11]. Indeed, in this population, VF measures have been successfully linked to those brain networks supporting both executive functions and language both in vivo [12,13,14,15,16,17] and at a neuropathological level [18]. Consistently, their utility has been proven either as individual screeners [9] or when included within second-level cognitive batteries [6]. Remarkably, VF measures have been also shown to be associated with patients’ motor and functional outcomes [19,20,21,22] and are acknowledged as sensitive indices of post-deep brain stimulation CI [23]. In addition, since VF tests are brief and require only verbal responses, they are suitable for fatigable patients and they are not affected by upper-limb disabilities, making them highly feasible in PD [24].
As highlighted by the Movement Disorders Society (MDS) [25, 26], there is a need for disease-specific clinimetric studies that address those tests that have been historically shown to be appropriate for detecting CI in PD, as is the case for VF. Such investigations would increase their level of recommendation for use in clinical practice and research [27]. Indeed, after a given test is made available to the clinical and scientific community and standardized in the normotypical population, clinimetric evidence in target patient cohorts should be always provided in order to improve users’ confidence in employing that test in real life, either clinical or research settings [27]. Additionally, with specific regards to test norms, it has been shown that cut-offs derived in normotypical populations—i.e., normality thresholds—might be poorly sensitive and, at variance, disproportionately biased towards specificity [28, 29]. Due to the detrimental entailments of such a stance towards clinical and research practice, researchers in the field of cognitive testing often commit to provide users with disease-specific cut-offs [28,29,30,31,32,33,34,35,36,37]—at least with regard to those brain disorders that are among the most prevalent and incident, as is the case for PD [35,36,37,38,39].
As to the Italian scenario, only two studies by Biundo et al. [38, 39] have to this day focused on the clinimetrics of VF tests in PD patients and have shown that both phonemic and semantic VF tests (PVF; SVF) are diagnostically sound for detecting CI in this population. However, these studies [38, 39] referred to an outdated normative dataset—i.e., that delivered by Novelli et al. [40] in 1986—and, most unfortunately, preceded the availability of the Alternate Verbal Fluency Battery (AVFB), standardized by Costa et al. [41] in 2013. Indeed, Costa et al. [41] not only provided updated norms for PVF and SVF test, but also normed, for the first time in Italy, an alternate phonemic-semantic VF task (AVF) —whose clinical utility in PD has been known for decades [42,43,44] and was recently demonstrated in Italy too [24]. AVF tests in fact represent a suitable alternative to much more common measures of set-shifting abilities that are, however, biased by PD patients’ motor disabilities—such as the Trail-Making Test-B [44]. This stance also happens to be supported by the fact that AVF tasks have been included in PD-specific cognitive screening batteries—such as the Parkinson Neuropsychometric Dementia Assessment [43] and the Parkinson’s Disease Cognitive Rating Scale [44].
Given the above premises, the primary scope of this study was to assess the construct validity, factor structure and internal consistency of Costa et al.’s [41] AVFB, as well as to examine its diagnostic properties, in an Italian cohort of non-demented PD patients. Moreover, the current investigation was aimed at deriving, from the abovementioned patient cohort, disease-specific cut-offs for Costa et al.’s [41] AVFB that could be used by Italian practitioners and clinical researchers.
Methods
Participants
Data on N = 192 Italian-speaking PD patients were retrospectively collected (126 males and 66 females; mean age = 62.6 ± 9.8 years; mean education = 12.1 ± 4.1 years). Patients were diagnosed with idiopathic PD (disease duration range = 3–20 months), between 2018 and 2023, according to the UK Brain Bank Criteria [45] by a team of expert neurologists through anamnestic interviews, neurological assessments, neuroradiological examinations and neuropsychological testing. Subjects were on medication during the cognitive assessment. Patients were free of PD-unrelated psychiatric disorders as well as of dementia—according to DSM-V criteria for a major neurocognitive disorder due to PD [46].
Materials
Patients were assessed with the AVFB by Costa et al. [41], which includes three subtests, namely the PVF, the SVF and the AVF. Cues for the PVF were the letters F, A and S, whilst those for the SVF were colors, animals and fruits. The AVF requires examinees to continuously alternate letter and category-cued words as follows: A—colors; F—animals; and S—fruits. In all of the above subtests, patients were given 60 s for each trial and instructed not to produce proper nouns, place names, numbers, or inflected words with the same suffix. The order of presentation was the following: (1) PVF, (2) SVF and (3) AVF. A Composite Shifting Index (CSI) reflecting the cost of switching from a single-cued VF task to a double-cued VF task was then calculated as follows: AVF/[(PVF + SVF)/2].
In addition, for clinical purposes, patients were assessed using either the Mini-Mental State Examination (MMSE) [47] or the Montreal Cognitive Assessment (MoCA) [48]. Because the vast majority of patients were administered the MoCA (73.4%), those who underwent the MMSE (N = 53) had their MMSE scores converted into MoCA ones via the equating algorithm by Aiello et al. [49]. Such a conversion proved to be fully valid, since the same two patients who scored below the cut-off on the MMSE [47] were also classified as impaired by the derived MoCA scores [48], with no further discrepancies being noted (Cohen’s k = 1; p < 0.001).
Statistics
Outcome measure
In the context of construct validity and diagnostic analyses, the MoCA was considered as an outcome, both in accordance with the MDS recommendations to use this test for screening purposes in this population [50] and given that the MoCA is widely known to highly load on executive-based cognitive processes [48], as is the case with VF tests [51]. In support to such an approach, the MoCA has already been used earlier as an outcome measure for testing the validity and diagnostics of executive-loaded tests in both normotypical [48, 52] and extrapyramidal populations [31, 35].
Psychometrics
Both AVFB and MoCA scores are normally distributed, as indexed by skewness and kurtosis values <|1| and |3|, respectively [53]. Hence, the construct validity of each VFB measures was tested against the MoCA via Bonferroni-corrected Pearson’s correlation coefficients. The effect size of correlation coefficients was classified as follows: (1) 0.10 < rs ≤ 0.30 → small; (2) 0.30 < rs ≤ 0.50 → medium; (3) rs > 0.50 → large [54]. Internal reliability and factorial validity of the AVFB were assessed via Cronbach’s α coefficient and a principal component analysis (PCA), respectively, by addressing all of its subtests—i.e., PVF, SVF, AVF and CSI scores.
Diagnostics
In order to test the diagnostics of each AVFB subtests, receiver-operating characteristic (ROC) analyses were carried out by operationalizing the positive state as an age- and education-adjusted MoCA score below or equal to the inner tolerance limit of the current Italian normative dataset [48]. Indeed, this threshold gives a safety level of at least 95% that least 95% of the normotypical population works beyond it [55, 56]. For those subtests yielding an acceptable AUC value (i.e., ≥ 0.70) [57], sensitivity, specificity and positive/negative likelihood ratios were then calculated at the optimal cut-off value identified by means of the Youden’s index. Positive likelihood ratio values ≥ 2 and negative likelihood ratio ones ≤ 0.5 were deemed as optimal [58]. Standardized positive/negative predictive values [58] were calculated by assuming that the prevalence of cognitive impairment in the PD population is 40% based on recent meta-analytical evidence [1]. The Number Needed for Screening Utility (NNSU) was then computed—with values ≤ 1.02 meaning that less than ≈1 individual needs to be screened for the test to be useful in the view of ruling-in/ruling-out the presence of the target condition [59]. For the purpose of these analyses, PVF, SVF, AVF and CSI scores were adjusted for significant demographic confounders based on Costa et al.’s [41] normative dataset.
Software
Analyses were carried out with IBM® SPSS® 27, R 4.3 (https://cran.r-project.org/) and jamovi 2.3 (https://www.jamovi.org/). The significance threshold was set at = 0.05 and Bonferroni-corrected whenever necessary.
Results
Table 1 summarizes patients’ demographic and cognitive measures.
Psychometrics
At αadjusted = 0.013, MoCA scores proved to be strongly associated with PVF (r(192) = 0.53; p < 0.001), SVF (r(192) = 0.51; p < 0.001) and AVF (r(192) = 0.54; p < 0.001) scores, whilst moderately with the CSI (r(192) = 0.34; p < 0.001).
The AVFB was internally reliable (Cronbach’s α = 0.77), with item-rest correlation coefficients ranging from 0.49 to 0.76; however, a nine-point increase in reliability could be obtained by dropping the CSI (Cronbach’s α = 0.86)—with the same not being true for remaining measures. Consistently, the PCA yielded a mono-component structure accounting for 67.65% of the variance (loading range = 0.67–0.97). However, when the CSI was exploratively dropped from the PCA, the AVFB retained its mono-component structure, although an increase in both explained variance (79.44%) and average loading size (range = 0.0.88–0.90) was detected.
Diagnostics
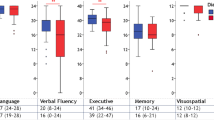
Ten out of 192 patients (5.2%) performed below the inner tolerance limit on the MoCA. In identifying such patients, adjusted PVF, SVF and AVF scores were highly accurate, whilst the CSI yielded an unacceptable AUC value (Fig. 1). Disease-specific cut-offs and associated diagnostic metrics were therefore only calculated for PVF, SVF and AVF tests, resulting overall optimal (Table 2). According to these thresholds, 33% of the sample was classified as impaired on the PVF, 24% on the SVF and 39% on the AVF.
ROC curves for demographically adjusted AVFB measures. ROC, receiver-operating characteristics; AVFB, Alternate Verbal Fluency Battery; PVF, Phonemic Verbal Fluency; SVF, Semantic Verbal Fluency; AVF, Alternate Verbal Fluency; CSI, Composite Shifting Index. PVF: AUC = 0.91; SE = 0.03; CI 95% [0.84, 0.97]; SVF: AUC = 0.85; SE = 0.05; CI 95% [0.75, 0.94]; AVF: AUC = 0.84; SE = 0.04; CI 95% [0.76, 0.92]; CSI: AUC = 0.58; SE = 0.11; CI 95% [0.37, 0.79]. AVFB measures were demographically adjusted according to Costa et al. [41]
Discussion
The present study provides Italian practitioners and clinical researchers with relevant psychometric and diagnostic information on Costa et al.’s [41] AVFB in non-demented PD patients.
In the current study, the AVFB proved to be internally consistent and valid at both the factorial and construct levels. However, it was found that by removing the CSI from the VFB, there was an improvement in its internal reliability as well as the extent to which the VFB itself fitted the underlying factor structure. Consistently, albeit significant, the correlation between the CSI and the MoCA was found to be weaker than that between the other AVFB subtests and the MoCA itself. Such results, together with the fact that the CSI, unlike the other AVFB subtests, was both able to distinguish PD patients with CI from those without CI, suggest that this measure has little or no clinical utility in this population.
Importantly, this report demonstrates the diagnostic value of PVF, SVF and AVF tests in non-demented PD patients. As to the PVF and the SVF, the present results align with the relevant literature by further confirming the usefulness of these tests for detecting CI in this population [24; 38; 39]. Additionally, it has been herewith shown, for the first time in Italy, that the AVF too is a diagnostically sound test to the abovementioned aim. This is consistent with previous reports supporting the use of the AVF as a motor-free measure of set-shifting abilities in PD [24, 42,43,44].
Moreover, the abovementioned findings are consistent with earlier neuroradiological reports showing, in this population, an association between VF performances and both the involvement of striatal structures [14, 16]—i.e., the neural hallmark of PD—and the integrity of networks supporting language [12, 15, 17] and attentive-executive processes [44].
In addition, the present report supports the notion that VF tests are appropriate screening tools in this population [10], as evidenced by the fact that PVF, SVF and AVF tests were characterized by optimal NNSU values.
Relevantly, the current study provides Italian clinicians and researchers with disease-specific cut-offs for PVF, SVF and AVF tests, which can be used for detecting CI after adjusting the raw scores on such tests for significant demographic confounders on the basis of Costa et al.’s [41] normative dataset.
Of course, this study is not free from limitations. First, due to its retrospective nature, no functional or motor data could be retrieved. Therefore, it was not possible to assess the extent to which such variables might have affected VF performance. Such an issue is most evident when referred to dysarthric features which, as already outlined in the Italian scenario, could bias the results of timed tasks requiring verbal responses [60].
To account for this issue, a promising tool might lie in the Verbal Fluency Index (VFI), a measure originally developed to control for the confounding effect of dysarthria on VF performance in amyotrophic lateral sclerosis (ALS) [61]. Since the VFI has been recently normed for the Italian population and evaluated for its clinimetrics in ALS [62], it is recommended that future studies attempt to investigate its feasibility in PD as well. Second, it should be noted that the prevalence of CI in the current cohort was particularly low (i.e., 5.2%) than that proposed in a recent meta-analysis on the subject (i.e., 40%) [1]. Therefore, further research aimed at confirming the diagnostic power of the AVFB in a more balanced PD cohort is needed. Relatedly, the present reports did not include demented patients, who should be the focus of future investigations on the AVFB. Third, since this study was retrospective, it only assessed the reliability of the VFB at an internal level. Therefore, future studies should also focus on examining its inter-rater and test–retest reliability. Fourth, the target condition was herewith operationalized using a first-level test—i.e., the MoCA: in order to be able to continue to use the current state of knowledge, it is desirable that future reports also use extensive second-level cognition to diagnose AFB examine battery.
In addition to the above, it makes sense to mention further, practically relevant information for future investigations. First, repeated-measure studies that explore the longitudinal feasibility of VFB in PD are still needed. This also includes the derivation of thresholds for defining clinically significant changes. Such investigations would be of great interest for at least two reasons: first, because VF tests are known to be able to detect involutionary trends in cognition after deep brain stimulation surgery [23]; second, because such measures have proved promising in predicting the onset of dementia in this population [63]. Furthermore, given the clear benefits that remote cognitive testing can offer when referred to patients with motor disabilities [64], it is desirable that future studies assess the clinimetrics and feasibility of the telephone-based version of Costa et al.’s [41] AVFB, which has been recently standardized for the Italian population [65].
In conclusion, the present study confirms that Costa et al.’s [41] AVFB, and more specifically its PVF, SVF and AVF subtests, is reliable, valid, and diagnostically sound instruments to detect CI in non-demented PD patients. Their use is therefore recommended in clinical practice and research.
Data availability
Datasets related to the present study are available upon reasonable request from interested researchers.
References
Baiano C, Barone P, Trojano L, Santangelo G (2020) Prevalence and clinical aspects of mild cognitive impairment in Parkinson’s disease: a meta-analysis. Mov Disord 35:45–54
Rosenthal E, Brennan L, Xie S, Hurtig H, Milber J, Weintraub D et al (2010) Association between cognition and function in patients with Parkinson disease with and without dementia. Mov Disord 25:1170–1176
Marras C, Rochon P, Lang AE (2002) Predicting motor decline and disability in Parkinson disease: a systematic review. Arch Neurol 59:1724–1728
Pedersen KF, Larsen JP, Tysnes OB, Alves G (2013) Prognosis of mild cognitive impairment in early Parkinson disease: the Norwegian ParkWest study. JAMA Neurol 70:580–586
Oosterveld LP, Allen JC Jr, Reinoso G, Seah SH, Tay KY, Au WL, Tan LC (2015) Prognostic factors for early mortality in Parkinson’s disease. Parkinsonism Relat Disord 21:226–230
Litvan I, Goldman JG, Tröster AI, Schmand BA, Weintraub D, Petersen RC et al (2012) Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: movement Disorder Society Task Force guidelines. Mov Disord 27:349–356
Henry JD, Crawford JR (2004) Verbal fluency deficits in Parkinson’s disease: a meta-analysis. J Int Neuropsychol Soc 10:608–622
Koerts J, Meijer HA, Colman KS, Tucha L, Lange KW, Tucha O (2013) What is measured with verbal fluency tests in Parkinson’s disease patients at different stages of the disease? J Neural Transm 120:403–411
Pfeiffer HCV, Løkkegaard A, Zoetmulder M, Friberg L, Werdelin L (2014) Cognitive impairment in early-stage non-demented Parkinson’s disease patients. Acta Neurol Scand 129:307–318
Torralva T, Laffaye T, Báez S, Gleichgerrcht E, Bruno D, Chade A et al (2015) Verbal fluency as a rapid screening test for cognitive impairment in early Parkinson’s disease. J Neuropsychiatry Clin Neurosci 27:244–247
Cholerton BA, Poston KL, Yang L, Rosenthal LS, Dawson TM, Pantelyat A et al (2021) Semantic fluency and processing speed are reduced in non-cognitively impaired participants with Parkinson’s disease. J Clin Exp Neuropsychol 43:469–480
Pereira JB, Junqué C, Martí MJ, Ramirez-Ruiz B, Bartres-Faz D, Tolosa E (2009) Structural brain correlates of verbal fluency in Parkinson’s disease. NeuroReport 20:741–744
Pagonabarraga J, Corcuera-Solano I, Vives-Gilabert Y, Llebaria G, García-Sánchez C, Pascual-Sedano B et al (2013) Pattern of regional cortical thinning associated with cognitive deterioration in Parkinson’s disease. PLoS ONE 8:e54980
Ellfolk U, Joutsa J, Rinne JO, Parkkola R, Jokinen P, Karrasch M (2014) Striatal volume is related to phonemic verbal fluency but not to semantic or alternating verbal fluency in early Parkinson’s disease. J Neural Transm 121:33–40
Magdalinou NK, Golden HL, Nicholas JM, Witoonpanich P, Mummery CJ, Morris HR et al (2018) Verbal adynamia in parkinsonian syndromes: behavioral correlates and neuroanatomical substrate. Neurocase 24:204–212
Rodriguez-Porcel F, Wilmskoetter J, Cooper C, Taylor JA, Fridriksson J, Hickok G, Bonilha L (2021) The relationship between dorsal stream connections to the caudate and verbal fluency in Parkinson disease. Brain Imaging Behav 15:2121–2125
Yang J, McMahon KL, Copland DA, Pourzinal D, Byrne GJ, Angwin AJ et al (2022) Semantic fluency deficits and associated brain activity in Parkinson’s disease with mild cognitive impairment. Brain Imaging Behav 16:2445–2456
El-Nazer R, Adler CH, Beach TG, Belden CM, Artz J, Shill HA et al (2019) Regional neuropathology distribution and verbal fluency impairments in Parkinson’s disease. Parkinsonism Relat Disord 65:73–78
Camicioli R, Oken BS, Sexton G, Kaye JA, Nutt JG (1998) Verbal fluency task affects gait in Parkinson’s disease with motor freezing. J Geriatr Psychiatry Neurol 11:181–185
Amboni M, Cozzolino A, Longo K, Picillo M, Barone P (2008) Freezing of gait and executive functions in patients with Parkinson’s disease. Mov Disord 23:395–400
Muslimović D, Post B, Speelman JD, Schmand B, de Haan RJ (2008) Determinants of disability and quality of life in mild to moderate Parkinson disease. Neurology 70:2241–2247
Smulders K, van Nimwegen M, Munneke M, Bloem BR, Kessels RP, Esselink RA (2013) Involvement of specific executive functions in mobility in Parkinson’s disease. Parkinsonism Relat Disord 19:126–128
Højlund A, Petersen MV, Sridharan KS, Østergaard K (2017) Worsening of verbal fluency after deep brain stimulation in Parkinson’s disease: a focused review. Comput Struct Biotechnol J 15:68–74
Ferrucci R, Mameli F, Ruggiero F, Reitano M, Miccoli M, Gemignani A et al (2022) Alternate fluency in Parkinson’s disease: a machine learning analysis. PLoS ONE 17:e0265803
Goldman JG, Holden S, Ouyang B, Bernard B, Goetz CG, Stebbins GT (2015) Diagnosing PD-MCI by MDS task force criteria: how many and which neuropsychological tests? Mov Disord 30:402–406
Hoogland J, van Wanrooij LL, Boel JA, Goldman JG, Stebbins GT, Dalrymple-Alford JC et al (2018) Detecting mild cognitive deficits in Parkinson’s disease: comparison of neuropsychological tests. Mov Disord 33:1750–1759
Aiello EN, Rimoldi S, Bolognini N, Appollonio I, Arcara G (2022) Psychometrics and diagnostics of Italian cognitive screening tests: a systematic review. Neurol Sci 43:821–845
Ilardi CR, Menichelli A, Michelutti M, Cattaruzza T, Manganotti P (2023) Optimal MoCA cutoffs for detecting biologically-defined patients with MCI and early dementia. Neurol Sci 44:159–170
Salvadori E, Cova I, Mele F, Pomati S, Pantoni L (2022) Prediction of post-stroke cognitive impairment by Montreal Cognitive Assessment (MoCA) performances in acute stroke: comparison of three normative datasets. Aging Clin Exp Res 34:1855–1863
Aiello EN, Solca F, Torre S, Carelli L, Ferrucci R, Priori A et al (2022) Diagnostics and clinical usability of the Montreal Cognitive Assessment (MoCA) in amyotrophic lateral sclerosis. Front Psychol 13:1012632
Solca F, Aiello EN, Migliore S, Torre S, Carelli L, Ferrucci R et al (2022) Diagnostic properties of the Frontal Assessment Battery (FAB) in Huntington’s disease. Front Psychol 13:1031871
Aiello EN, Solca F, Torre S, Carelli L, Ferrucci R, Priori A et al (2023) Feasibility and diagnostics of the Frontal Assessment Battery (FAB) in amyotrophic lateral sclerosis. Neurol Sci 44:587–592
Aiello EN, Verde F, Milone I, Giacopuzzi Grigoli E, Dubini A, Carelli L et al (2022) The Frontal Assessment Battery (FAB) effectively discriminates between MCI and dementia within the clinical spectrum of neurochemically confirmed Alzheimer’s disease. Front Psychol 13:1054321
Aiello EN, Solca F, Torre S, Lafronza A, Maranzano A, Bonetti R et al (2023) Validity, diagnostics and feasibility of the Italian version of the Montreal Cognitive Assessment (MoCA) in Huntington’s disease. Neurol Sci 1–8
Aiello EN, D’Iorio A, Solca F, Torre S, Bonetti R, Scheveger F et al (2023) Clinimetrics and feasibility of the Italian version of the Frontal Assessment Battery (FAB) in non-demented Parkinson’s disease patients. J Neural Transm 130(5):687–696
D’Iorio A, Aiello EN, Amboni M, Vitale C, Verde F, Silani V et al (2023) Validity and diagnostics of the Italian version of the Montreal Cognitive Assessment (MoCA) in non-demented Parkinson’s disease patients. Aging Clin Exp Res 35:2157–2163
Terruzzi S, Funghi G, Meli C, Barozzi N, Zappini F, Papagno C, Dodich A (2023) The FACE test: a new neuropsychological task to assess the recognition of complex mental states from faces. Neurol Sci 1–9
Biundo R, Weis L, Pilleri M, Facchini S, Formento-Dojot P, Vallelunga A, Antonini A (2013) Diagnostic and screening power of neuropsychological testing in detecting mild cognitive impairment in Parkinson’s disease. J Neural Transm 120:627–633
Biundo R, Weis L, Facchini S, Formento-Dojot P, Vallelunga A, Pilleri M, Antonini A (2014) Cognitive profiling of Parkinson disease patients with mild cognitive impairment and dementia. Parkinsonism Relat Disord 20:394–399
Novelli G, Papagno C, Capitani E, Laiacona M, Cappa SF, Vallar G (1986) Tre test clinici di ricerca e produzione lessicale. Taratura su sogetti normali. Arch Psicol Neurol Psichiatr 47:278–296
Costa A, Bagoj E, Monaco M, Zabberoni S, De Rosa S, Papantonio AM et al (2014) Standardization and normative data obtained in the Italian population for a new verbal fluency instrument, the phonemic/semantic alternate fluency test. Neurol Sci 35:365–372
Downes JJ, Sharp HM, Costall BM, Sagar HJ, Howe J (1993) Alternating fluency in Parkinson’s disease: an evaluation of the attentional control theory of cognitive impairment. Brain 116:887–902
Kalbe E, Calabrese P, Kohn N, Hilker R, Riedel O, Wittchen HU et al (2008) Screening for cognitive deficits in Parkinson’s disease with the Parkinson neuropsychometric dementia assessment (PANDA) instrument. Parkinsonism Relat Disord 14:93–101
Pagonabarraga J, Kulisevsky J, Llebaria G, García-Sánchez C, Pascual-Sedano B, Gironell A (2008) Parkinson’s disease-cognitive rating scale: a new cognitive scale specific for Parkinson’s disease. Mov Disord 23:998–1005
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184
American Psychiatric Association (2013) Diagnostic and statistical manual of mentaldisorders (5th ed).
Carpinelli-Mazzi M, Iavarone A, Russo G, Musella C, Milan G, D’Anna F et al (2020) Mini-Mental State Examination: new normative values on subjects in Southern Italy. Aging Clin Exp Res 32:699–702
Aiello EN, Gramegna C, Esposito A, Gazzaniga V, Zago S, Difonzo T et al (2022) The Montreal Cognitive Assessment (MoCA): updated norms and psychometric insights into adaptive testing from healthy individuals in Northern Italy. Aging Clin Exp Res 34:375–382
Aiello EN, Pasotti F, Appollonio I, Bolognini N (2022) Equating Mini-Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA) scores: conversion norms from a healthy Italian population sample. Aging Clin Exp Res 34:1721–1724
Skorvanek M, Goldman JG, Jahanshahi M, Marras C, Rektorova I, Schmand B et al (2018) Global scales for cognitive screening in Parkinson’s disease: critique and recommendations. Mov Disord 33:208–218
Aita SL, Beach JD, Taylor SE, Borgogna NC, Harrell MN, Hill BD (2018) Executive, language, or both? An examination of the construct validity of verbal fluency measures. Appl Neuropsychol Adult 26:441–451
Aiello EN, Esposito A, Appollonio I, Bolognini N (2022) Diagnostic properties of the Frontal Assessment Battery (FAB) in Italian healthy adults. Aging Clin Exp Res 34:1021–1026
Kim HY (2013) Statistical notes for clinical researchers: assessing normal distribution (2) using skewness and kurtosis. Restor Dent Endod 38:52–54
Cohen J (1988) Statistical power analysis for the behavioral sciences. New York, NY: Routledge Academic.
Capitani E, Laiacona M (2017) Outer and inner tolerance limits: their usefulness for the construction of norms and the standardization of neuropsychological tests. Clin Neuropsychol 31:1219–1230
Aiello EN, Depaoli EG (2022) Norms and standardizations in neuropsychology via equivalent scores: software solutions and practical guides. Neurol Sci 43:961–966
Larner AJ (2021) Measures not directly related to the 2 x 2 contingency table. In: Larner AJ (ed) The 2x2 matrix. contingency, confusion, and the metrics of binary classification. pp 113–132
Larner AJ (2021) Paired measures. In: Larner AJ (ed) The 2x2 matrix. contingency, confusion, and the metrics of binary classification. pp 15–48
Larner AJ (2019) New unitary metrics for dementia test accuracy studies. Prog Neurol Psychiatry 23:21–25
Carelli L, Solca F, Migliore S, Torre S, Brugnera A, Mancini F et al (2021) Compensating for verbal-motor deficits in neuropsychological assessment in movement disorders: sensitivity and specificity of the ECAS in Parkinson’s and Huntington’s diseases. Neurol Sci 42:4997–5006
Abrahams S, Leigh PN, Harvey A, Vythelingum GN, Grise D, Goldstein LH (2000) Verbal fluency and executive dysfunction in amyotrophic lateral sclerosis (ALS). Neuropsychologia 38:734–747
Canu E, Castelnovo V, Rancoita PM, Leocadi M, Lamanuzzi A, Spinelli EG, Agosta, F (2023) Italian reference values and brain correlates of verbal fluency index–vs standard verbal fluency test–to assess executive dysfunction in ALS. Amyotrophic LateralSclerosis and Frontotemporal Degeneration. 1–9
Jacobs DM, Marder K, Cote LJ, Sano M, Stern Y, Mayeux R (1995) Neuropsychological characteristics of preclinical dementia in Parkinson’s disease. Neurology 45:1691–1696
Zanin E, Aiello EN, Diana L, Fusi G, Bonato M, Niang A et al (2022) Tele-neuropsychological assessment tools in Italy: a systematic review on psychometric properties and usability. Neurol Sci 43:125–138
Aiello EN, Preti AN, Pucci V, Diana L, Corvaglia A, di San Pietro CB, N. et al (2022) The Italian telephone-based Verbal Fluency Battery (t-VFB): standardization and preliminary clinical usability evidence. Front Psychol 13:963164
Acknowledgements
Roberta Ferrucci was supported by “Aldo Ravelli” Center for Neurotechnology and Experimental Brain Therapeutics, Department of Health Sciences, University of Milan, Milano, Italy
Funding
Open access funding provided by Università degli Studi di Milano within the CRUI-CARE Agreement. This research was partially funded by the Italian Ministry of Health to the Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico and to the IRCCS Istituto Auxologico Italiano.
Ministero della Salute
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
V. S. received compensation for consulting services and/or speaking activities from AveXis, Cytokinetics, Italfarmaco, Liquidweb S.r.l., Novartis Pharma AG and Zambon and receives or has received research supports from the Italian Ministry of Health, AriSLA and E-Rare Joint Transnational Call. He is in the Editorial Board of Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration, European Neurology, American Journal of Neurodegenerative Diseases, Frontiers in Neurology and Exploration of Neuroprotective Therapy. B.P. received compensation for consulting services and/or speaking activities from Liquidweb S.r.l. B.P is the Associated Editor for Frontiers in Neuroscience. N. T. received compensation for consulting services from Amylyx Pharmaceuticals and Zambon Biotech SA. He is the Associate Editor for Frontiers in Aging Neuroscience.
Ethical approval
All subjects provided informed consent. The protocol and procedures were approved by the local Institutional Review Board. The procedures were conducted in accordance with the Declaration of Helsinki.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aiello, E.N., Mameli, F., Ruggiero, F. et al. Psychometrics and diagnostics of the Italian version of the Alternate Verbal Fluency Battery (AVFB) in non-demented Parkinson’s disease patients. Neurol Sci (2024). https://doi.org/10.1007/s10072-024-07436-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10072-024-07436-5