Abstract
Background
Blood-based biomarkers for Alzheimer’s disease (AD) are promising to be used in clinical settings. The liver is an important degradation organ of the body. Whether liver function affects the levels of AD biomarkers needs to be studied.
Objective
To investigate the associations between liver function and the plasma levels of AD biomarkers.
Methods
We conducted an ADNI cohort-based cross-sectional study. Thirteen liver function markers commonly used in clinical settings were analyzed: total protein (TP), albumin (AL), globulin (GL), AL/GL ratio (A/G), total bilirubin (TB), direct bilirubin (DB), indirect bilirubin (IB), alanine aminotransferase (ALT), aspartate aminotransferase (AST), AST/ALT ratio, alkaline phosphatase (ALP), lactate dehydrogenase (LDH), and γ-glutamyltransferase (GGT). Liquid chromatography-tandem mass spectrometry was used to detect the plasma Aβ42 and Aβ40 concentrations. Single Molecule array technique was used to measure the plasma p-tau181 and NfL concentrations. We used linear regression models to analyze the associations between liver function markers and the levels of AD plasma biomarkers.
Results
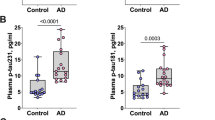
ALP was positively associated with the levels of plasma Aβ42 (β = 0.16, P = 0.018) and Aβ40 (β = 0.21, P = 0.004). LDH was positively associated with the levels of plasma p-tau181 (β = 0.09, P = 0.022). While NfL was correlated with multiple liver function markers, including AL, A/G, ALT, AST/ALT, and LDH.
Conclusion
Liver function was associated with the plasma levels of AD biomarkers. It needs to consider the potential influence of liver function on the reference ranges and the interpretation of results for AD biomarkers before clinical use.


Similar content being viewed by others
Data availability
The data used in this study are openly available on the ADNI website at www.adni-info.org.
References
Gustavsson A et al (2022) Global estimates on the number of persons across the Alzheimer’s disease continuum. Alzheimers Dement. https://doi.org/10.1002/alz.12694
Jack CRJ et al (2013) Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol 12(2):207–216. https://doi.org/10.1016/S1474-4422(12)70291-0
Jack CJ et al (2018) NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14(4):535–562. https://doi.org/10.1016/j.jalz.2018.02.018
Teunissen CE et al (2022) Blood-based biomarkers for Alzheimer’s disease: towards clinical implementation. Lancet Neurol 21(1):66–77. https://doi.org/10.1016/S1474-4422(21)00361-6
Hansson O et al (2022) The Alzheimer’s Association appropriate use recommendations for blood biomarkers in Alzheimer’s disease. Alzheimers Dement 18(12):2669–2686. https://doi.org/10.1002/alz.12756
Hampel H et al (2018) Blood-based biomarkers for Alzheimer disease: mapping the road to the clinic. Nat Rev Neurol 14(11):639–652. https://doi.org/10.1038/s41582-018-0079-7
Bassendine MF et al (2020) Is Alzheimer’s disease a liver disease of the brain? J Alzheimers Dis 75(1):1–14. https://doi.org/10.3233/JAD-190848
Cheng Y, Tian D, Wang Y (2020) Peripheral clearance of brain-derived Aβ in Alzheimer’s disease: pathophysiology and therapeutic perspectives. Transl Neurodegener 9(1):16. https://doi.org/10.1186/s40035-020-00195-1
Cheng Y et al (2023) Physiological beta-amyloid clearance by the liver and its therapeutic potential for Alzheimer’s disease. Acta Neuropathol. https://doi.org/10.1007/s00401-023-02559-z
Peng Z et al (2022) Association of liver disease with brain volume loss, cognitive decline, and plasma neurodegenerative disease biomarkers. Neurobiol Aging 120:34–42. https://doi.org/10.1016/j.neurobiolaging.2022.08.004
Wang YR et al (2017) Associations between hepatic functions and plasma amyloid-beta levels-implications for the capacity of liver in peripheral amyloid-beta clearance. Mol Neurobiol 54(3):2338–2344. https://doi.org/10.1007/s12035-016-9826-1
Mueller SG et al (2005) Ways toward an early diagnosis in Alzheimer’s disease: the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Alzheimers Dement 1(1):55–66
Kang JH et al (2015) The Alzheimer’s disease neuroimaging initiative 2 biomarker core: a review of progress and plans. Alzheimers Dement 11(7):772–791. https://doi.org/10.1016/j.jalz.2015.05.003
Ovod V et al (2017) Amyloid beta concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimers Dement 13(8):841–849. https://doi.org/10.1016/j.jalz.2017.06.2266
Karikari TK et al (2021) Diagnostic performance and prediction of clinical progression of plasma phospho-tau181 in the Alzheimer’s Disease Neuroimaging Initiative. Mol Psychiatry 26(2):429–442. https://doi.org/10.1038/s41380-020-00923-z
Mattsson N et al (2019) Association between longitudinal plasma neurofilament light and neurodegeneration in patients with Alzheimer disease. JAMA Neurol 76(7):791–799. https://doi.org/10.1001/jamaneurol.2019.0765
Landau SM et al (2013) Comparing positron emission tomography imaging and cerebrospinal fluid measurements of beta-amyloid. Ann Neurol 74(6):826–836. https://doi.org/10.1002/ana.23908
Nho K et al (2019) Association of altered liver enzymes with Alzheimer disease diagnosis, cognition, neuroimaging measures, and cerebrospinal fluid biomarkers. JAMA Netw Open 2(7):e197978. https://doi.org/10.1001/jamanetworkopen.2019.7978
Roberts KF et al (2014) Amyloid-beta efflux from the central nervous system into the plasma. Ann Neurol 76(6):837–844. https://doi.org/10.1002/ana.24270
Goodman JR et al (2018) Characterization of dural sinus-associated lymphatic vasculature in human Alzheimer’s dementia subjects. Brain Behav Immun 73:34–40. https://doi.org/10.1016/j.bbi.2018.07.020
Shibata M et al (2000) Clearance of Alzheimer’s amyloid-ss(1–40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest 106(12):1489–1499. https://doi.org/10.1172/JCI10498
Storck SE et al (2016) Endothelial LRP1 transports amyloid-beta(1–42) across the blood-brain barrier. J Clin Invest 126(1):123–136. https://doi.org/10.1172/JCI81108
Ghiso J et al (2004) Systemic catabolism of Alzheimer’s Abeta40 and Abeta42. J Biol Chem 279(44):45897–45908. https://doi.org/10.1074/jbc.M407668200
Tamaki C et al (2006) Major involvement of low-density lipoprotein receptor-related protein 1 in the clearance of plasma free amyloid beta-peptide by the liver. Pharm Res 23(7):1407–1416. https://doi.org/10.1007/s11095-006-0208-7
Yamashita M et al (1989) Measurement of serum alkaline phosphatase isozyme I in brain-damaged patients. Neurol Med Chir 29(11):995–998. https://doi.org/10.2176/nmc.29.995. (Tokyo)
Kellett KA et al (2011) Plasma alkaline phosphatase is elevated in Alzheimer’s disease and inversely correlates with cognitive function. Int J Mol Epidemiol Genet 2(2):114–121
Long DM et al (2020) Lactate dehydrogenase expression modulates longevity and neurodegeneration in Drosophila melanogaster. Aging 12(11):10041–10058. https://doi.org/10.18632/aging.103373. (Albany NY)
Kapogiannis D, Mattson MP (2011) Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer’s disease. Lancet Neurol 10(2):187–198. https://doi.org/10.1016/S1474-4422(10)70277-5
Zhang B et al (2023) Effect of renal function on the diagnostic performance of plasma biomarkers for Alzheimer’s disease. Front Aging Neurosci 15. https://doi.org/10.3389/fnagi.2023.1150510
Acknowledgements
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association, Alzheimer’s Drug Discovery Foundation, Araclon Biotech, BioClinica, Inc., Biogen, BristolMyers Squibb Company, CereSpir, Inc., Cogstate; Eisai Inc., Elan Pharmaceuticals, Inc., Eli Lilly and Company, EuroImmun, F.Hoffmann-La Roche Ltd., and its affiliated company Genentech, Inc., Fujirebio, GE Healthcare, IXICO Ltd., Janssen Alzheimer Immunotherapy Research and Development, LLC., Johnson & Johnson Pharmaceutical Research and Development, LLC., Lumosity, Lundbeck; Merck & Co., Inc., Meso Scale Diagnostics, LLC., NeuroRx Research, Neurotrack Technologies, Novartis Pharmaceuticals Corporation, Pfizer Inc., Piramal Imaging, Servier, Takeda Pharmaceutical Company, and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuroimaging at the University of Southern California.
Funding
This work was supported by the National Key R&D Program of China (Grant No. 2022YFC2010103) and the Central health research project (Grant No. 2020ZD10).
Author information
Authors and Affiliations
Consortia
Contributions
Bin Zhang: study design, analysis of data, and manuscript drafting. Cheng Zhang: study design and statistical analysis. Yu YeWang and Lei AnChen: revision of manuscript. Ya NanQiao, YuWang and Dang TaoPeng: study design and revision of manuscript. All authors reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
All data used in the study was from the ADNI cohort. The ADNI cohort was authorized by the local institutional review committee and conducted in accordance with the Declaration of Helsinki.
Informed consent
Informed consent was obtained from all subjects involved in the ADNI cohort.
Conflict of interest
The authors declare no competing interests.
Disclaimer
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in the analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wpcontent/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, B., Zhang, C., Wang, Y. et al. Associations of liver function with plasma biomarkers for Alzheimer’s Disease. Neurol Sci 45, 2625–2631 (2024). https://doi.org/10.1007/s10072-023-07284-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-07284-9