Abstract
Objective
This study aimed to examine the volumes of thalamic nuclei and the intrinsic thalamic network in patients with Wilson's disease (WDs), and to explore the correlation between these volumes and the severity of neurological symptoms.
Methods
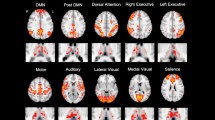
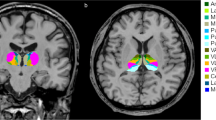
A total of 61 WDs and 33 healthy controls (HCs) were included in the study. The volumes of 25 bilateral thalamic nuclei were measured using structural imaging analysis with Freesurfer, and the intrinsic thalamic network was evaluated through structural covariance network (SCN) analysis.
Results
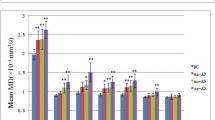
The results indicated that multiple thalamic nuclei were smaller in WDs compared to HCs, including mediodorsal medial magnocellular (MDm), anterior ventral (AV), central median (CeM), centromedian (CM), lateral geniculate (LGN), limitans-suprageniculate (L-Sg), reuniens-medial ventral (MV), paracentral (Pc), parafascicular (Pf), paratenial (Pt), pulvinar anterior (PuA), pulvinar inferior (PuI), pulvinar medial (PuM), ventral anterior (VA), ventral anterior magnocellular (VAmc), ventral lateral anterior (VLa), ventral lateral posterior (VLp), ventromedial (VM), ventral posterolateral (VPL), and right middle dorsal intralaminar (MDI). The study also found a negative correlation between the UWDRS scores and the volume of the right MDm. The intrinsic thalamic network analysis showed abnormal topological properties in WDs, including increased mean local efficiency, modularity, normalized clustering coefficient, small-world index, and characteristic path length, and a corresponding decrease in mean node betweenness centrality. WDs with cerebral involvement had a lower modularity compared to HCs.
Conclusions
The findings suggest that the majority of thalamic nuclei in WDs exhibit significant volume reduction, and the atrophy of the right MDm is closely related to the severity of neurological symptoms. The intrinsic thalamic network in WDs demonstrated abnormal topological properties, indicating a close relationship with neurological impairment.






Similar content being viewed by others
Data availability
The data supporting the findings of this study can be obtained upon request from the corresponding author, Wenbin Hu.
References
Członkowska A, Litwin T, Dusek P, Ferenci P, Lutsenko S, Medici V, Rybakowski JK, Weiss KH, Schilsky ML (2018) Wilson disease. Nat Rev Dis Primers 4(1):21
Guindi M (2019) Wilson disease. Semin Diagn Pathol 36(6):415–422
Bandmann O, Weiss KH, Kaler SG (2015) Wilson’s disease and other neurological copper disorders. Lancet Neurol 14(1):103–113
Pfeiffer RF (2016) Wilson disease. Continuum (Minneapolis, Minn.) 22(4 Movement Disorders):1246–1261
Smolinski L, Ziemssen T, Akgun K, Antos A, Skowrońska M, Kurkowska-Jastrzębska I, Członkowska A, Litwin T (2022) Brain atrophy is substantially accelerated in neurological Wilson’s disease: a longitudinal study. Mov Disord 37(12):2446–2451
Ge X, Wang L, Pan L, Ye H, Zhu X, Fan S, Feng Q, Yu W, Ding Z (2022) Amplitude of low-frequency fluctuation after a single-trigger pain in patients with classical trigeminal neuralgia. J Headache Pain 23(1):117
Yu XE, Gao S, Yang RM, Han YZ (2019) MR imaging of the brain in neurologic Wilson disease. AJNR Am J Neuroradiol 40(1):178–183
Litwin T, Dzieżyc K, Karliński M, Chabik G, Czepiel W, Członkowska A (2015) Early neurological worsening in patients with Wilson’s disease. J Neurol Sci 355(1–2):162–167
Zou L, Song Y, Zhou X, Chu J, Tang X (2019) Regional morphometric abnormalities and clinical relevance in Wilson’s disease. Mov Disord 34(4):545–554
Shribman S, Bocchetta M, Sudre CH, Acosta-Cabronero J, Burrows M, Cook P, Thomas DL, Gillett GT, Tsochatzis EA, Bandmann O, Rohrer JD, Warner TT (2022) Neuroimaging correlates of brain injury in Wilson’s disease: a multimodal, whole-brain MRI study. Brain 145(1):263–275
Wang A, Wu H, Xu C, Tang L, Lee J, Wang M, Jiang M, Li C, Lu Q, Zhang C (2017) Study on lesion assessment of cerebello-thalamo-cortical network in Wilson’s disease with diffusion tensor imaging. Neural Plast 2017:7323121
Bullmore ET, Bassett DS (2011) Brain graphs: graphical models of the human brain connectome. Annu Rev Clin Psychol 7:113–140
Wang P, Li W, Zhu H, Liu X, Yu T, Zhang D, Zhang Y (2022) Reorganization of the brain structural covariance network in ischemic moyamoya disease revealed by graph theoretical analysis. Front Aging Neurosci 14:788661
Alexander-Bloch A, Raznahan A, Bullmore E, Giedd J (2013) The convergence of maturational change and structural covariance in human cortical networks. J Neurosci 33(7):2889–2899
Li S, Bai R, Yang Y, Zhao R, Upreti B, Wang X, Liu S, Cheng Y, Xu J (2022) Abnormal cortical thickness and structural covariance networks in systemic lupus erythematosus patients without major neuropsychiatric manifestations. Arthritis Res Ther 24(1):259
Hong W, Li M, Liu Z, Li X, Huai H, Jia D, Jin W, Zhao Z, Liu L, Li J, Sun F, Xu R, Zhao Z (2021) Heterogeneous alterations in thalamic subfields in major depression disorder. J Affect Disord 295:1079–1086
Iglesias JE, Insausti R, Lerma-Usabiaga G, Bocchetta M, Van Leemput K, Greve DN, van der Kouwe A, Fischl B, Caballero-Gaudes C, Paz-Alonso PM (2018) A probabilistic atlas of the human thalamic nuclei combining ex vivo MRI and histology. Neuroimage 183:314–326
Hou W, Zheng SJ, Duan Z (2022) Interpretation of the 2022 edition guidelines for hepatolenticular degeneration diagnosis and treatment. Zhonghua Gan Zang Bing Za Zhi 30(3):276–278
Leinweber B, Möller JC, Scherag A, Reuner U, Günther P, Lang CJG, Schmidt HHJ, Schrader C, Bandmann O, Czlonkowska A, Oertel WH, Hefter H (2008) Evaluation of the Unified Wilson’s Disease Rating Scale (UWDRS) in German patients with treated Wilson’s disease. Mov Disord 23(1):54–62
Hosseini SM, Hoeft F, Kesler SR (2012) GAT: a graph-theoretical analysis toolbox for analyzing between-group differences in large-scale structural and functional brain networks. PLoS ONE 7(7):e40709
Coppola P, Allanson J, Naci L, Adapa R, Finoia P, Williams GB, Pickard JD, Owen AM, Menon DK, Stamatakis EA (2022) The complexity of the stream of consciousness. Commun Biol 5(1):1173
Watts DJ, Strogatz SH (1998) Collective dynamics of “small-world” networks. Nature 393(6684):440–442
Girvan M, Newman MEJ (2002) Community structure in social and biological networks. Proc Natl Acad Sci USA 99(12):7821–7826
Bordes S, Werner C, Mathkour M, McCormack E, Iwanaga J, Loukas M, Lammle M, Dumont AS, Tubbs RS (2020) Arterial supply of the thalamus: a comprehensive review. World neurosurgery 137:310–318
Bosch-Bouju C, Hyland BI, Parr-Brownlie LC (2013) Motor thalamus integration of cortical, cerebellar and basal ganglia information: implications for normal and parkinsonian conditions. Front Comput Neurosci 7:163
Neychev VK, Fan X, Mitev VI, Hess EJ, Jinnah HA (2008) The basal ganglia and cerebellum interact in the expression of dystonic movement. Brain 131(Pt 9):2499–2509
Silveri MC (2021) Contribution of the cerebellum and the basal ganglia to language production: speech, word fluency, and sentence construction-evidence from pathology. Cerebellum (London, England) 20(2):282–294
Lipat AL, Clark DJ, Hass CJ, Cruz-Almeida Y (2022) Gait subgroups among older adults with chronic pain differ in cerebellum and basal ganglia gray matter volumes. Exp Gerontol 163:111773
Barthélemy M (2004) Betweenness centrality in large complex networks. Eur Phys J B 38(2):163–168
Bullmore E, Sporns O (2009) Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10(3):186–198
Saetia S, Yoshimura N, Koike Y (2021) Constructing brain connectivity model using causal network reconstruction approach. Front Neuroinform 15:619557
Brier MR, Thomas JB, Fagan AM, Hassenstab J, Holtzman DM, Benzinger TL, Morris JC, Ances BM (2014) Functional connectivity and graph theory in preclinical Alzheimer’s disease. Neurobiol Aging 35(4):757–768
Kocevar G, Stamile C, Hannoun S, Cotton F, Vukusic S, Durand-Dubief F, Sappey-Marinier D (2016) Graph theory-based brain connectivity for automatic classification of multiple sclerosis clinical courses. Front Neurosci 10:478
Lee HJ, Seo SA, Lee BI, Kim SE, Park KM (2020) Thalamic nuclei volumes and network in juvenile myoclonic epilepsy. Acta Neurol Scand 141(4):271–278
Anastasiades PG, Collins DP, Carter AG (2021) Mediodorsal and ventromedial thalamus engage distinct L1 circuits in the prefrontal cortex. Neuron 109(2):314-330.e4
Acknowledgements
The authors would like to thank Zonagxian Yao and Congming Xu for their assistance in data collecting.
Funding
This work was supported by 2022QNJJ04(Kunshan Traditional Chinese Medicine Hospital Youth Science and Technology Fund) and 2021sfyle01 (Anhui University of Chinese Medicine).
Author information
Authors and Affiliations
Contributions
Bing Zhang and Guang Yang contributed to the conception and organization of the research project. Bing Zhang contributed to the execution of the research project. Bing zhang, Rong Zhang, Chunyang Xu performed the statistical analysis and drafted the manuscript. Wenbin Hu and Xiaogang He critically reviewed the manuscript for important intellectual content. Wenbin Hu provided funding. All authors approved the final version of the manuscript before submission.
Corresponding author
Ethics declarations
Ethical approval
The study received approval from all authors. It was conducted in accordance with the Declaration of, Helsinki, and approved by the Ethics Committee of the Affiliated Hospital of the Institute of Neurology, Anhui University of Chinese Medicine (Ethics Approval No. 2021-Lun-Zi (13), translated from Chinese).
Informed consent
Participants were informed of the program and they put their signature on informed consent form.
Conflict of interest
All authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Co-first authorship: Bing Zhang and Guang Yang.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, B., Yang, G., Xu, C. et al. The volume and structural covariance network of thalamic nuclei in patients with Wilson’s disease: an investigation of the association with neurological impairment. Neurol Sci 45, 2063–2073 (2024). https://doi.org/10.1007/s10072-023-07245-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-07245-2