Abstract
Purpose
To study the value of 3.0T magnetic resonance imaging with diffusion tensor imaging (DTI) and 3D-arterial spin labeling (ASL) perfusion imaging in the diagnosis of the crossed cerebellar diaschisis (CCD) after the unilateral supratentorial subacute cerebral hemorrhage.
Methods
Fifty-eight patients with the unilateral supratentorial subacute cerebral hemorrhage who underwent diffusion tensor imaging (DTI), 3D-arterial spin labeling (ASL), and conventional magnetic resonance imaging (MRI) scanning were enrolled. Cerebral blood flow (CBF) values of the perihematomal edema (PHE) and bilateral cerebellar hemisphere were measured on ASL mapping, and the fractional anisotropy (FA) and mean diffusivity (MD) values of the bilateral cortical, pontine, and middle cerebellar peduncle (MCP) were measured on DTI mapping.
Results
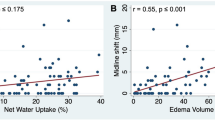
In the CCD(+) group, FA values of the cerebral cortex and pontine ipsilateral to the lesion were statistically reduced compared to the contralateral side (P < 0.05), and the FA and MD values of the middle cerebellar peduncle (MCP) contralateral to the lesion were statistically reduced compared to the ipsilateral side (P < 0.05). Positive correlation was detected between the CBF values of the perihematomal edema (PHE) and the CBF values of cerebellar hemispheres (r = 0.642, P < 0.05), whereas the CBF values of PHE had a significantly high positive correlation with the FA in the contralateral MCP (r = 0.854, P < 0.05). CBF values in the contralateral cerebellar hemisphere correlated with FA (r = 0.466, P < 0.05) and MD values (r = 0.718, P < 0.05) in the contralateral MCP.
Conclusion
Hemodynamic alterations of PHE and cortical-ponts-cerebellum (CPC) fibrous pathway damage are associated with the development of CCD; DTI technique can assess the degree of CPC fiber pathway injury at an early stage.





Similar content being viewed by others
Data availability
The processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.
References
Tripathi SM, Murray AD, Wischik CM, Schelter B (2022) Crossed cerebellar diaschisis in Alzheimer's disease. Nucl Med Commun 43(4):423–427. https://doi.org/10.1097/MNM.0000000000001531
Han S, Wang X, Xu K, Hu C (2016) Crossed cerebellar diaschisis: three case reports imaging using a tri-modality PET/CT-MR system. Medicine 95(2):e2526. https://doi.org/10.1097/MD.0000000000002526
Kang KM, Sohn CH, Choi SH, Jung KH, Yoo RE, Yun TJ et al (2017) Detection of crossed cerebellar diaschisis in hyperacute ischemic stroke using arterial spin-labeled MR imaging. PLoS One 12(3):e0173971. https://doi.org/10.1371/journal.pone.0173971
Sin DS, Kim MH, Park SA, Joo MC, Kim MS (2018) Crossed cerebellar diaschisis: risk factors and correlation to functional recovery in intracerebral hemorrhage. Ann Rehabil Med 42(1):8–17. https://doi.org/10.5535/arm.2018.42.1.8
Jiang S, Li X, Li Z, Chang X, Chen Y, Huang Y et al (2020) Cerebello-cerebral connectivity in idiopathic generalized epilepsy. Eur Radiol 30(7):3924–3933. https://doi.org/10.1007/s00330-020-06674-3
Brodal P, Bjaalie JG (1997) Salient anatomic features of the cortico-ponto-cerebellar pathway. Prog Brain Res 114:227–249. https://doi.org/10.1016/s0079-6123(08)63367-1
Agrawal KL, Mittal BR, Bhattacharya A, Khandelwal N, Prabhakar S (2011) Crossed cerebellar diaschisis on F-18 FDG PET/CT. Indian J Nucl Med 26(2):102–103
Ma J, Zhao L, Yuan K, Yan J, Zhang Y, Zhu J et al (2022) Crossed cerebellar diaschisis after acute ischemic stroke detected by intravoxel incoherent motion magnetic resonance imaging. Neurol Sci 43(2):1135–1141. https://doi.org/10.1007/s10072-021-05425-6
Chen S, Guan M, Lian HJ, Ma LJ, Shang JK, He S et al (2014) Crossed cerebellar diaschisis detected by arterial spin-labeled perfusion magnetic resonance imaging in subacute ischemic stroke. J Stroke Cerebrovasc Dis 23(9):2378–2383. https://doi.org/10.1016/j.jstrokecerebrovasdis.2014.05.009
Lee S, Na Y, Tae WS, Pyun SB (2020) Clinical and neuroimaging factors associated with aphasia severity in stroke patients: diffusion tensor imaging study. Sci Rep 10(1):12874. https://doi.org/10.1038/s41598-020-69741-1
Chaudhary N, Pandey AS, Gemmete JJ, Hua Y, Huang Y, Gu Y et al (2015) Diffusion tensor imaging in hemorrhagic stroke. Exp Neurol 272:88–96. https://doi.org/10.1016/j.expneurol.2015.05.011
van der Eerden AW, van den Heuvel TL, Perlbarg V, Vart P, Vos PE, Puybasset L, Galanaud D, Platel B, Manniesing R, Goraj BM (2021) Traumatic cerebral microbleeds in the subacute phase are practical and early predictors of abnormality of the normal-appearing white matter in the chronic phase. AJNR Am J Neuroradiol 42(5):861–867. https://doi.org/10.3174/ajnr.A7028
Koyama T, Tsuji M, Nishimura H, Miyake H, Ohmura T, Domen K (2013) Diffusion tensor imaging for intracerebral hemorrhage outcome prediction: comparison using data from the corona radiata/internal capsule and the cerebral peduncle. J Stroke Cerebrovasc Dis 22(1):72–79. https://doi.org/10.1016/j.jstrokecerebrovasdis.2011.06.014
Koyama T, Uchiyama Y, Domen K (2018) Associations of diffusion-tensor fractional anisotropy and FIM outcome assessments after intracerebral hemorrhage. J Stroke Cerebrovasc Dis 27(10):2869–2876. https://doi.org/10.1016/j.jstrokecerebrovasdis.2018.06.012
Xu X, Chen X, Zhang J, Zheng Y, Sun G, Yu X et al (2014) Comparison of the Tada formula with software slicer: precise and low-cost method for volume assessment of intracerebral hematoma. Stroke 45(11):3433–3435. https://doi.org/10.1161/STROKEAHA.114.007095
Lin DD, Kleinman JT, Wityk RJ, Gottesman RF, Hillis AE, Lee AW et al (2009) Crossed cerebellar diaschisis in acute stroke detected by dynamic susceptibility contrast MR perfusion imaging. AJNR Am J Neuroradiol 30(4):710–715. https://doi.org/10.3174/ajnr.A1435
Broderick JP, Grotta JC, Naidech AM, Steiner T, Sprigg N, Toyoda K et al (2021) The story of intracerebral hemorrhage: from recalcitrant to treatable disease. Stroke 52(5):1905–1914. https://doi.org/10.1161/STROKEAHA.121.033484
Naccarato M, Ajčević M, Furlanis G, Lugnan C, Buoite Stella A, Scali I et al (2020) Novel quantitative approach for crossed cerebellar diaschisis detection in acute ischemic stroke using CT perfusion. J Neurol Sci 416:117008. https://doi.org/10.1016/j.jns.2020.117008
Joya A, Padro D, Gómez-Vallejo V, Plaza-García S, Llop J, Martín A (2018) PET imaging of crossed cerebellar diaschisis after long-term cerebral ischemia in rats. Contrast Media Mol Imaging 2018:2483078
Ryu H, Park CH (2020) Structural characteristic of the arcuate fasciculus in patients with fluent aphasia following intracranial hemorrhage: a diffusion tensor tractography study. Brain Sci 10(5):280. https://doi.org/10.3390/brainsci10050280
Park CH, Ryu H, Kim CH, Joa KL, Kim MO, Jung HY (2020) Injury of corticospinal tract in a patient with subarachnoid hemorrhage as determined by diffusion tensor tractography: a case report. Brain Sci 10(3):177. https://doi.org/10.3390/brainsci10030177
Park CH, Kim SH, Jung HY (2020) Diffusion-tensor-tractography-based diagnosis for injury of corticospinal tract in a patient with hemiplegia following traumatic brain injury. Diagnostics (Basel, Switzerland) 10(3):156. https://doi.org/10.3390/diagnostics10030156
Shi K, Tian DC, Li ZG, Ducruet AF, Lawton MT, Shi FD (2019) Global brain inflammation in stroke. Lancet Neurol 18(11):1058–1066. https://doi.org/10.1016/S1474-4422(19)30078-X
Fan SJ, Lee FY, Cheung MM, Ding AY, Yang J, Ma SJ et al (2013) Bilateral substantia nigra and pyramidal tract changes following experimental intracerebral hemorrhage: an MR diffusion tensor imaging study. NMR Biomed 26(9):1089–1095. https://doi.org/10.1002/nbm.2922
Palesi F, De Rinaldis A, Castellazzi G, Calamante F, Muhlert N, Chard D et al (2017) Contralateral cortico-ponto-cerebellar pathways reconstruction in humans in vivo: implications for reciprocal cerebro-cerebellar structural connectivity in motor and non-motor areas. Sci Rep 7(1):12841. https://doi.org/10.1038/s41598-017-13079-8
Chow BW, Nuñez V, Kaplan L, Granger AJ, Bistrong K, Zucker HL et al (2020) Caveolae in CNS arterioles mediate neurovascular coupling. Nature 579(7797):106–110. https://doi.org/10.1038/s41586-020-2026-1
Kaplan L, Chow BW, Gu C (2020) Neuronal regulation of the blood-brain barrier and neurovascular coupling. Nat Rev Neurosci 21(8):416–432. https://doi.org/10.1038/s41583-020-0322-2
Boltze J, Ferrara F, Hainsworth AH, Bridges LR, Zille M, Lobsien D et al (2019) Lesional and perilesional tissue characterization by automated image processing in a novel gyrencephalic animal model of peracute intracerebral hemorrhage. J Cereb Blood Flow Metab 39(12):2521–2535. https://doi.org/10.1177/0271678X18802119
Venkat P, Chopp M, Chen J (2016) New insights into coupling and uncoupling of cerebral blood flow and metabolism in the brain. Croat Med J 57(3):223–228. https://doi.org/10.3325/cmj.2016.57.223
Giezendanner S, Fisler MS, Soravia LM, Andreotti J, Walther S, Wiest R et al (2016) Microstructure and cerebral blood flow within white matter of the human brain: a TBSS analysis. PLoS One 11(3):e0150657. https://doi.org/10.1371/journal.pone.0150657
Yin X, Zhou Y, Yan S, Lou M (2019) Effects of cerebral blood flow and white matter integrity on cognition in CADASIL patients. Front Psychiatry 9:741. https://doi.org/10.3389/fpsyt.2018.00741
Bouhrara M, Alisch JSR, Khattar N, Kim RW, Rejimon AC, Cortina LE et al (2020) Association of cerebral blood flow with myelin content in cognitively unimpaired adults. BMJ Neurol Open 2(1):e000053. https://doi.org/10.1136/bmjno-2020-000053
Tarumi T, Thomas BP, Wang C, Zhang L, Liu J, Turner M et al (2019) Ambulatory pulse pressure, brain neuronal fiber integrity, and cerebral blood flow in older adults. J Cereb Blood Flow Metab 39(5):926–936. https://doi.org/10.1177/0271678X17745027
Zhang Y, Wang X, Cheng J, Lin Y, Yang L, Cao Z et al (2019) Changes of fractional anisotropy and RGMa in crossed cerebellar diaschisis induced by middle cerebral artery occlusion. Exp Ther Med 18(5):3595–3602. https://doi.org/10.3892/etm.2019.7986
Tae WS, Ham BJ, Pyun SB, Kang SH, Kim BJ (2018) Current clinical applications of diffusion-tensor imaging in neurological disorders. J Clin Neurol 14(2):129–140. https://doi.org/10.3988/jcn.2018.14.2.129
Funding
This work was supported by the Science and Technology Innovative Development Foundation of Taian (2020NS249), and the Science and technology projects of Shandong Geriatrics Society (LKJGG2021W092).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval
The studies involving human participants were reviewed and approved by the Second Affiliated Hospital of Shandong First Medical University Ethics Committee. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual's legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Conflict of interest
The authors declare no competing interests.
Informed consent
All authors were informed and agreed to publish the paper.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Q., Zhang, Y., Shi, Q. et al. Application study of DTI combined with ASL in the crossed cerebellar diaschisis after subacute cerebral hemorrhage. Neurol Sci 44, 3949–3956 (2023). https://doi.org/10.1007/s10072-023-06908-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-06908-4