Abstract
Background and purpose
Intracerebral and subarachnoid hemorrhage are critical conditions with a high mortality, and the outcome for the individual patient is notoriously difficult to predict. Biomarkers that reflect disease severity and predict outcome are therefore warranted.
Methods
Blood samples from 40 patients with intracerebral, 46 patients with subarachnoid hemorrhage, and 70 healthy individuals were collected. Levels of glial fibrillary acidic protein (GFAP) and neuroglobin were measured by ultra-sensitive single molecule array and enzyme-linked immunosorbent assay, respectively. Clinical information including mortality and functional outcome was recorded.
Results
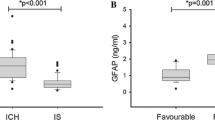
Blood levels of GFAP and neuroglobin in intracerebral and subarachnoid hemorrhage patients were significantly elevated when compared to healthy individuals (all p < 0.0001). GFAP levels were significantly higher in patients dying or with poor functional outcome than in healthy individuals (all p ≤ 0.01). GFAP levels separated survivors from non-survivors with an area under receiver operating characteristics (AUROC) = 0.78 (confidence interval (CI) 0.59–0.98) for intracerebral hemorrhage and 0.82 (CI 0.69–0.94) for subarachnoid hemorrhage patients. The Akaike and Bayesian information criteria (AIC/BIC) for mortality/poor outcome prediction improved when combining GFAP levels with hematoma volume (p = 0.04/p < 0.01), National Institutes of Health Stroke Scale (NIHSS) (p = 0.09/p < 0.01), Hunt-Hess (p < 0.05/p = 0.21), or Fischer score (p < 0.05/p = 0.02).
Conclusions
Elevated GFAP levels at admission to hospital predicted mortality and poor outcome in our cohort of intracerebral and subarachnoid hemorrhage patients. Neuroglobin levels did not provide additional information. Combining GFAP measurements with clinical disease severity scores increased outcome prediction precision. This may suggest that GFAP measurement could improve prognostication in patients with intracerebral or subarachnoid hemorrhage.
Registration: This sub-trial was not registered.



Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to patient confidentiality and local data management laws, but are available from the corresponding author upon reasonable request.
References
Unnithan AKA, M Das J, Mehta P (2022) Hemorrhagic stroke. In: StatPearls [Internet] StatPearls Publishing, Treasure Island. Available from: https://www.ncbi.nlm.nih.gov/books/NBK559173/
Huttner HB, Schellinger PD, Hartmann M, Kohrmann M, Juettler E, Wikner J et al (2006) Hematoma growth and outcome in treated neurocritical care patients with intracerebral hemorrhage related to oral anticoagulant therapy: comparison of acute treatment strategies using vitamin K, fresh frozen plasma, and prothrombin complex concentrates. Stroke 37(6):1465–1470
Maher M, Schweizer TA, Macdonald RL (2020) Treatment of spontaneous subarachnoid hemorrhage: guidelines and gaps. Stroke 51(4):1326–1332
Cordonnier C, Demchuk A, Ziai W, Anderson CS (2018) Intracerebral haemorrhage: current approaches to acute management. Lancet 392(10154):1257–1268
Glushakova OY, Glushakov AV, Miller ER, Valadka AB, Hayes RL (2016) Biomarkers for acute diagnosis and management of stroke in neurointensive care units. Brain Circ 2(1):28–47
Yang Z, Wang KK (2015) Glial fibrillary acidic protein: from intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci 38(6):364–374
Dvorak F, Haberer I, Sitzer M, Foerch C (2009) Characterisation of the diagnostic window of serum glial fibrillary acidic protein for the differentiation of intracerebral haemorrhage and ischaemic stroke. Cerebrovasc Dis 27(1):37–41
Foerch C, Curdt I, Yan B, Dvorak F, Hermans M, Berkefeld J et al (2006) Serum glial fibrillary acidic protein as a biomarker for intracerebral haemorrhage in patients with acute stroke. J Neurol Neurosurg Psychiatry 77(2):181–184
Foerch C, Niessner M, Back T, Bauerle M, De Marchis GM, Ferbert A et al (2012) Diagnostic accuracy of plasma glial fibrillary acidic protein for differentiating intracerebral hemorrhage and cerebral ischemia in patients with symptoms of acute stroke. Clin Chem 58(1):237–245
Stanca DM, Marginean IC, Soritau O, Dragos C, Marginean M, Muresanu DF et al (2015) GFAP and antibodies against NMDA receptor subunit NR2 as biomarkers for acute cerebrovascular diseases. J Cell Mol Med 19(9):2253–2261
Xiong L, Yang Y, Zhang M, Xu W (2015) The use of serum glial fibrillary acidic protein test as a promising tool for intracerebral hemorrhage diagnosis in Chinese patients and prediction of the short-term functional outcomes. Neurol Sci 36(11):2081–2087
Llombart V, García-Berrocoso T, Bustamante A, Giralt D, Rodriguez-Luna D, Muchada M et al (2016) Plasmatic retinol-binding protein 4 and glial fibrillary acidic protein as biomarkers to differentiate ischemic stroke and intracerebral hemorrhage. J Neurochem 136(2):416–424
Mattila OS, Ashton NJ, Blennow K, Zetterberg H, Harve-Rytsala H, Pihlasviita S et al (2021) Ultra-early differential diagnosis of acute cerebral ischemia and hemorrhagic stroke by measuring the prehospital release rate of GFAP. Clin Chem 67(10):1361–1372
Kedziora J, Burzynska M, Gozdzik W, Kubler A, Kobylinska K, Adamik B (2021) Biomarkers of neurological outcome after aneurysmal subarachnoid hemorrhage as early predictors at discharge from an intensive care unit. Neurocrit Care 34(3):856–866
Kedziora J Burzynska M Gozdzik W Kubler A Uryga A Kasprowicz M et al (2020) Brain-specific biomarkers as mortality predictors after aneurysmal subarachnoid haemorrhage J Clin Med 9(12)
Kaneda K, Fujita M, Yamashita S, Kaneko T, Kawamura Y, Izumi T et al (2010) Prognostic value of biochemical markers of brain damage and oxidative stress in post-surgical aneurysmal subarachnoid hemorrhage patients. Brain Res Bull 81(1):173–177
Petzold A, Keir G, Kerr M, Kay A, Kitchen N, Smith M et al (2006) Early identification of secondary brain damage in subarachnoid hemorrhage: a role for glial fibrillary acidic protein. J Neurotrauma 23(7):1179–1184
Luyckx E, Van Acker ZP, Ponsaerts P, Dewilde S (2019) Neuroglobin expression models as a tool to study its function. Oxid Med Cell Longev 2019:5728129
Qiu XY, Chen XQ (2014) Neuroglobin - recent developments. Biomol Concepts 5(3):195–208
Cai H, Zheng S, Cai B, Yao P, Ding C, Chen F et al (2018) Neuroglobin as a novel biomarker for predicting poor outcomes in aneurysmal subarachnoid hemorrhage. World Neurosurg 116:e258–e265
Ding CY, Kang DZ, Wang ZL, Lin YX, Jiang CZ, Yu LH et al (2019) Serum Ngb (neuroglobin) is associated with brain metabolism and functional outcome of aneurysmal subarachnoid hemorrhage. Stroke 50(7):1887–1890
Lauridsen SV, Hvas AM, Sandgaard E, Gyldenholm T, Rahbek C, Hjort N et al (2018) Coagulation profile after spontaneous intracerebral hemorrhage: a cohort study. J Stroke Cerebrovasc Dis 27(11):2951–2961
Lauridsen SV, Hvas CL, Sandgaard E, Gyldenholm T, Mikkelsen R, Obbekjaer T et al (2019) Thromboelastometry shows early hypercoagulation in patients with spontaneous subarachnoid hemorrhage. World Neurosurg 130:e140–e149
Hviid CVB, Gyldenholm T, Lauridsen SV, Hjort N, Hvas AM, Parkner T (2020) Plasma neurofilament light chain is associated with mortality after spontaneous intracerebral hemorrhage. Clin Chem Lab Med 58(2):261–267
Hviid CVB, Lauridsen SV, Gyldenholm T, Sunde N, Parkner T, Hvas AM (2020) Plasma neurofilament light chain is associated with poor functional outcome and mortality rate after spontaneous subarachnoid hemorrhage. Transl Stroke Res 11(4):671–677
Saver JL, Chaisinanunkul N, Campbell BCV, Grotta JC, Hill MD, Khatri P et al (2021) Standardized nomenclature for modified Rankin scale global disability outcomes: consensus recommendations from Stroke Therapy Academic Industry Roundtable XI. Stroke 52(9):3054–3062
Brott T, Adams HP Jr, Olinger CP, Marler JR, Barsan WG, Biller J et al (1989) Measurements of acute cerebral infarction: a clinical examination scale. Stroke 20(7):864–870
Kwak R, Kadoya S, Suzuki T (1983) Factors affecting the prognosis in thalamic hemorrhage. Stroke 14(4):493–500
Moons KG, de Groot JA, Linnet K, Reitsma JB, Bossuyt PM (2012) Quantifying the added value of a diagnostic test or marker. Clin Chem 58(10):1408–1417
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44(3):837–845
Unden J, Strandberg K, Malm J, Campbell E, Rosengren L, Stenflo J et al (2009) Explorative investigation of biomarkers of brain damage and coagulation system activation in clinical stroke differentiation. J Neurol 256(1):72–77
Ehrenreich H, Kastner A, Weissenborn K, Streeter J, Sperling S, Wang KK et al (2011) Circulating damage marker profiles support a neuroprotective effect of erythropoietin in ischemic stroke patients. Mol Med 17(11–12):1306–1310
Vos PE, van Gils M, Beems T, Zimmerman C, Verbeek MM (2006) Increased GFAP and S100beta but not NSE serum levels after subarachnoid haemorrhage are associated with clinical severity. Eur J Neurol 13(6):632–638
Shemilt M, Boutin A, Lauzier F, Zarychanski R, Moore L, McIntyre LA et al (2019) Prognostic value of glial fibrillary acidic protein in patients with moderate and severe traumatic brain injury: a systematic review and meta-analysis. Crit Care Med 47(6):e522–e529
Bazarian JJ, Biberthaler P, Welch RD, Lewis LM, Barzo P, Bogner-Flatz V et al (2018) Serum GFAP and UCH-L1 for prediction of absence of intracranial injuries on head CT (ALERT-TBI): a multicentre observational study. Lancet Neurol 17(9):782–789
Funding
The study was graciously funded by Grosserer L.F. Foghts Fond and A.P. Møller Fonden. We also thank CLS Behring and Octapharma for unrestricted financial research support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval and informed consent
The study was performed in accordance with the Declaration of Helsinki and was approved by the Central Denmark Region Committees on Health Research Ethics (case no. 1–10-72–95-14 and 1–10-72–94-14). The Danish Data Agency (journal no. 2014–41-3416) approved the study before initiation. Informed consent was obtained from the patients when possible. If the cognitive state of the patient made informed consent impossible, consent was obtained from the patients’ next of kin and the patients’ general practitioner.
Conflict of interest
None of the financial donors had any influence on the design, analysis, interpretation of the results, or manuscript writing. None of the authors have any conflicts of interest to disclose regarding the present manuscript.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gyldenholm, T., Hvas, C.L., Hvas, AM. et al. Serum glial fibrillary acidic protein (GFAP) predicts outcome after intracerebral and subarachnoid hemorrhage. Neurol Sci 43, 6011–6019 (2022). https://doi.org/10.1007/s10072-022-06274-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-022-06274-7