Abstract
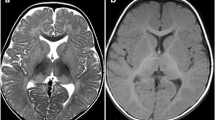
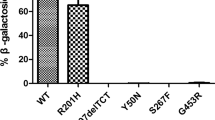
AB variant is the rarest form of GM2 gangliosidosis, neurodegenerative diseases caused by lysosomal accumulation of GM2 gangliosides. Less than thirty cases are referenced in the literature, and to date, no late-onset form has been described. Our proband is a 22-year-old male with spinocerebellar ataxia and lower limbs motor deficiency. His symptoms started at the age of 10. A genetic analysis revealed two mutations in the GM2A gene encoding the GM2 activator protein (GM2-AP), an essential co-factor of hexosaminidase A. Both mutations, GM2A:c.79A > T:p.Lys27* and GM2A:c.415C > T:p.Pro139Ser, were inherited respectively from his father and his mother. The nonsense mutation was predicted to be likely pathogenic, but the missense mutation was of unknown significance. To establish the pathogenicity of this variant, we studied GM2 accumulation and GM2A gene expression. Electron microscopy and immunofluorescence performed on patient’s fibroblasts did not reveal any lysosomal accumulation of GM2. There was also no difference in GM2A gene expression using RT-qPCR, and both mutations were found on cDNA Sanger sequencing. Measurement of plasma gangliosides by liquid-phase chromatography–tandem mass spectrometry showed an accumulation of GM2 in our patient’s plasma at 83.5 nmol/L, and a GM2/GM3 ratio at 0.066 (median of negative control at 30.2 nmol/L [19.7–46.8] and 0.019 respectively). Therefore, the association of both p.Lys27* and p.Pro169Ser mutations leads to a GM2-AP functional deficiency. Whereas the first mutation is more likely to be linked with infantile form of GM2 gangliosidosis, the hypomorphic p.Pro169Ser variant may be the first associated with a late-onset form of AB variant.




Similar content being viewed by others
References
Sandhoff K, Harzer K (2013) Gangliosides and gangliosidoses: principles of molecular and metabolic pathogenesis. J Neurosci 33(25):10195–10208. https://doi.org/10.1523/JNEUROSCI.0822-13.2013
Leal AF, Benincore-Flórez E, Solano-Galarza D, Garzón Jaramillo RG, Echeverri-Peña OY, Suarez DA, Alméciga-Díaz CJ, & Espejo-Mojica AJ. (2020). GM2 Gangliosidoses: clinical features, pathophysiological aspects, and current therapies. Int J Mol Sci 21(17). https://doi.org/10.3390/ijms21176213
Aureli M, Mauri L, Ciampa MG, Prinetti A, Toffano G, Secchieri C, Sonnino S (2016) GM1 ganglioside: past studies and future potential. Mol Neurobiol 53(3):1824–1842. https://doi.org/10.1007/s12035-015-9136-z
Yu RK, Tsai Y-T, Ariga T, Yanagisawa M (2011) Structures, biosynthesis, and functions of gangliosides—an overview. J Oleo Sci 60(10):537–544. https://doi.org/10.5650/jos.60.537
Schnaar RL (2019) The biology of gangliosides. Adv Carbohydr Chem Biochem 76:113–148. https://doi.org/10.1016/bs.accb.2018.09.002
Tropak MB, Yonekawa S, Karumuthil-Melethil S, Thompson P, Wakarchuk W, Gray SJ, Walia JS, Mark BL, Mahuran D (2016) Construction of a hybrid β-hexosaminidase subunit capable of forming stable homodimers that hydrolyze GM2 ganglioside in vivo. Mol Therapy Methods Clin Dev 3:15057. https://doi.org/10.1038/mtm.2015.57
Ikonne JU, Rattazzi MC, Desnick RJ (1975) Characterization of Hex S, the major residual beta hexosaminidase activity in type O Gm2 gangliosidosis (Sandhoff-Jatzkewitz disease). Am J Hum Genet 27(5):639–650
Mahuran DJ (1999) Biochemical consequences of mutations causing the GM2 gangliosidoses. Biochem Biophys Acta 1455(2–3):105–138. https://doi.org/10.1016/s0925-4439(99)00074-5
Bley AE, Giannikopoulos OA, Hayden D, Kubilus K, Tifft CJ, Eichler FS (2011) Natural history of infantile G(M2) gangliosidosis. Pediatrics 128(5):e1233-1241. https://doi.org/10.1542/peds.2011-0078
King KE, Kim S, Whitley CB, Jarnes-Utz JR (2020) The juvenile gangliosidoses: a timeline of clinical change. Mol Gen Metabolism Reports 25:100676. https://doi.org/10.1016/j.ymgmr.2020.100676
Masingue M, Dufour L, Lenglet T, Saleille L, Goizet C, Ayrignac X, Ory-Magne F, Barth M, Lamari F, Mandia D, Caillaud C, Nadjar Y (2020) Natural history of adult patients with GM2 gangliosidosis. Ann Neurol 87(4):609–617. https://doi.org/10.1002/ana.25689
Tay W. (1881). Symmetrical changes in the region of the yellow spot in each eye of an infant.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL, ACMG Laboratory Quality AssuranceCommittee (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Gen Med: Official J Am College Med Gen 17(5):405–424. https://doi.org/10.1038/gim.2015.30
Navon R, Baram D (1987) Depletion of cellular beta-hexosaminidase by imipramine is prevented by dexamethasone; implications for treating psychotic hexosaminidase-A deficient patients. Biochem Biophys Res Commun 148(3):1098–1103. https://doi.org/10.1016/s0006-291x(87)80245-0
Palmeri S, Mangano L, Battisti C, Malandrini A, Federico A (1992) Imipramine induced lipidosis and dexamethasone effect: morphological and biochemical study in normal and chronic GM2 gangliosidosis fibroblasts. J Neurol Sci 110(1–2):215–221. https://doi.org/10.1016/0022-510x(92)90030-o
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55(4):611–622. https://doi.org/10.1373/clinchem.2008.112797
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, & Speleman F. (2002). Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biology 3(7), research0034.1-research0034.11
Ferreira CR, Gahl WA (2017) Lysosomal storage diseases. Trans Sci Rare Dis 2(1–2):1–71. https://doi.org/10.3233/TRD-160005
Martins C, Brunel-Guitton C, Lortie A, Gauvin F, Morales CR, Mitchell GA, Pshezhetsky AV (2017) Atypical juvenile presentation of GM2 gangliosidosis AB in a patient compound-heterozygote for c.259G > T and c.164C > T mutations in the GM2A gene. Mol Gen Metabolism Reports 11:24–29. https://doi.org/10.1016/j.ymgmr.2017.01.017
Salih MA, Seidahmed MZ, El Khashab HY, Hamad MHA, Bosley TM, Burn S, Myers A, Landsverk ML, Crotwell PL, Bilguvar K, Mane S, Kruer MC (2015) Mutation in GM2A leads to a progressive chorea-dementia syndrome. Tremor and Other Hyperkinetic Movements (New York, NY) 5:306. https://doi.org/10.7916/D8D21WQ0
Schepers U, Glombitza G, Lemm T, Hoffmann A, Chabas A, Ozand P, Sandhoff K (1996) Molecular analysis of a GM2-activator deficiency in two patients with GM2-gangliosidosis AB variant. Am J Hum Genet 59(5):1048–1056
Schröder M, Schnabel D, Hurwitz R, Young E, Suzuki K, Sandhoff K (1993) Molecular genetics of GM2-gangliosidosis AB variant: a novel mutation and expression in BHK cells. Hum Genet 92(5):437–440. https://doi.org/10.1007/BF00216446
Xie B, Wang W, Mahuran DJ (1992) A Cys138-to-Arg substitution in the GM2 activator protein is associated with the AB variant form of GM2 gangliosidosis. Am J Hum Genet 50(5):1046–1052
Chen B, Rigat B, Curry C, Mahuran DJ (1999) Structure of the GM2A gene: identification of an exon 2 nonsense mutation and a naturally occurring transcript with an in-frame deletion of exon 2. Am J Hum Genet 65(1):77–87. https://doi.org/10.1086/302463
García-Moreno JF, Romão L (2020) Perspective in alternative splicing coupled to nonsense-mediated mRNA decay. Int J Mol Sci 21(24):9424. https://doi.org/10.3390/ijms21249424
Renaud D, Brodsky M (2016) GM2-gangliosidosis, AB variant: clinical, ophthalmological, MRI, and molecular findings. JIMD Reports 25:83–86. https://doi.org/10.1007/8904_2015_469
Brackmann F, Kehrer C, Kustermann W, Böhringer J, Krägeloh-Mann I, Trollmann R (2017) Rare variant of GM2 gangliosidosis through activator-protein deficiency. Neuropediatrics 48(2):127–130. https://doi.org/10.1055/s-0037-1598646
Sheth J, Datar C, Mistri M, Bhavsar R, Sheth F, Shah K (2016) GM2 gangliosidosis AB variant: novel mutation from India - a case report with a review. BMC Pediatr 16:88. https://doi.org/10.1186/s12887-016-0626-6
İnci A, Cengiz Ergin FB, Biberoğlu G, Okur İ, Ezgü FS, Tümer L (2021) Two patients from Turkey with a novel variant in the GM2A gene and review of the literature. J Pediatric Endocrinol Metabolism: JPEM 34(6):805–812. https://doi.org/10.1515/jpem-2020-0655
Neudorfer O, Pastores GM, Zeng BJ, Gianutsos J, Zaroff CM, Kolodny EH (2005) Late-onset Tay-Sachs disease: phenotypic characterization and genotypic correlations in 21 affected patients. Gen Med: Official J Am College Med Gen 7(2):119–123. https://doi.org/10.1097/01.gim.0000154300.84107.75
Navon R, Proia RL (1989) The mutations in Ashkenazi Jews with adult GM2 gangliosidosis the adult form of Tay-Sachs disease. Science (New York NY) 243(4897):1471–1474. https://doi.org/10.1126/science.2522679
Paw BH, Kaback MM, Neufeld EF (1989) Molecular basis of adult-onset and chronic GM2 gangliosidoses in patients of Ashkenazi Jewish origin: substitution of serine for glycine at position 269 of the alpha-subunit of beta-hexosaminidase. Proc Natl Acad Sci USA 86(7):2413–2417
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ganne, B., Dauriat, B., Richard, L. et al. GM2 gangliosidosis AB variant: first case of late onset and review of the literature. Neurol Sci 43, 6517–6527 (2022). https://doi.org/10.1007/s10072-022-06270-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-022-06270-x