Abstract
Background
Olfactory dysfunction is one of the earliest non-motor symptoms (NMS) in Parkinson’s disease (PD). There are contradictory results regarding the association of olfactory dysfunction and dopamine uptake in striatal nuclei among PD patients. It has been suggested that different motor subtypes of PD vary in the disease pathophysiology and progression. Thus, we hypothesized that there might be different associations between olfactory dysfunction and striatal dopaminergic neuronal loss among three motor subtypes of PD, namely, indeterminate, postural instability and gait difficulty (PIGD), and tremor-dominant (TD).
Methods
We recruited 162 healthy controls (HCs) and 464 drug-naïve PD patients from PPMI who underwent common PD scaling tests. Striatal binding ratios (SBRs) of DaTSCAN images in caudate and putamen nuclei were calculated. To assess the olfactory function, the University of Pennsylvania Smell Identification Test (UPSIT) was carried out.
Results
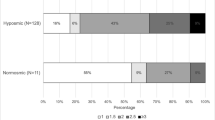
The UPSIT score was significantly correlated with MDS-UPDRS part I (p value: 0.002, correlation coefficient: − 0.160), MDS-UPDRS part III (p value: 0.000, correlation coefficient: − 0.248), and SBR score in right (p value: 0.000, correlation coefficient: 0.240) and left caudate (p value: 0.000, correlation coefficient: 0.221) and right (p value: 0.000, correlation coefficient: 0.323) and left putamen (p value: 0.000, correlation coefficient: 0.335) nucleus in TD subtype. There were no significant correlations in HC, PIGD, and indeterminate subjects.
Conclusion
The olfactory dysfunction was correlated with dopamine transporter activity in striatal nuclei only in the TD subtype. Therefore, the olfactory dysfunction in PIGD and indeterminate subtype may not be a predictive factor for the future decrease in dopamine uptake.

Similar content being viewed by others
Data availability
The datasets analyzed during the current study are available upon request with no restriction.
References
Hayes MT (2019) Parkinson’s disease and parkinsonism. Am J Med 132(7):802–807. https://doi.org/10.1016/j.amjmed.2019.03.001
Armstrong MJ, Okun MS (2020) Diagnosis and treatment of Parkinson disease: a review. JAMA 323(6):548–560. https://doi.org/10.1001/jama.2019.22360
Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24(2):197–211. https://doi.org/10.1016/s0197-4580(02)00065-9
Eek T, Larsson M, Dizdar N (2021) Odor recognition memory in Parkinson’s disease: a systematic review. Front Aging Neurosci 13:625171–625171. https://doi.org/10.3389/fnagi.2021.625171
Rossi M, Escobar AM, Bril A, Millar Vernetti P, De Palo JI, Cerquetti D, Merello M (2016) Motor features in Parkinson’s disease with normal olfactory function. Mov Disord 31(9):1414–1417. https://doi.org/10.1002/mds.26687
Yoo HS, Chung SJ, Lee YH, Ye BS, Sohn YH, Lee PH (2020) Association between olfactory deficit and motor and cognitive function in Parkinson’s disease. J Mov Disord 13(2):133–141. https://doi.org/10.14802/jmd.19082
He R, Zhao Y, He Y, Zhou Y, Yang J, Zhou X, Zhu L, Zhou X, Liu Z, Xu Q, Sun Q, Tan J, Yan X, Tang B, Guo J (2020) Olfactory dysfunction predicts disease progression in Parkinson’s disease a longitudinal study. Front Neurosci 14(1264). https://doi.org/10.3389/fnins.2020.569777
Lee DH, Oh JS, Ham JH, Lee JJ, Lee I, Lee PH, Kim JS, Sohn YH (2015) Is normosmic Parkinson disease a unique clinical phenotype? Neurology 85(15):1270–1275. https://doi.org/10.1212/wnl.0000000000001999
Qian E, Huang Y (2019) Subtyping of Parkinson’s disease - where are we up to? Aging Dis 10 (5) 1130–1139 https://doi.org/10.14336/AD.2019.0112
Jankovic J, McDermott M, Carter J, Gauthier S, Goetz C, Golbe L, Huber S, Koller W, Olanow C, Shoulson I, Stern M, Tanner C, Weiner W, Parkinson Study G (1990) Variable expression of Parkinson’s disease. Neurology 40(10):1529. https://doi.org/10.1212/WNL.40.10.1529
Nutt JG (2016) Motor subtype in Parkinson’s disease: different disorders or different stages of disease? Mov Disord 31(7):957–961. https://doi.org/10.1002/mds.26657
Ren J, Hua P, Li Y, Pan C, Yan L, Yu C, Zhang L, Xu P, Zhang M, Liu W (2020) Comparison of three motor subtype classifications in de novo Parkinson’s disease patients. Front Neurol 11:601225. https://doi.org/10.3389/fneur.2020.601225
De Pablo-Fernández E, Lees AJ, Holton JL, Warner TT (2019) Prognosis and neuropathologic correlation of clinical subtypes of Parkinson disease. JAMA Neurol 76(4):470–479. https://doi.org/10.1001/jamaneurol.2018.4377
Rezvanian S, Lockhart T, Frames C, Soangra R, Lieberman A (2018) Motor subtypes of Parkinson’s disease can be identified by frequency component of postural stability. Sensors (Basel) 18 (4). https://doi.org/10.3390/s18041102
Grahn JA, Parkinson JA, Owen AM (2009) The role of the basal ganglia in learning and memory: neuropsychological studies. Behav Brain Res 199(1):53–60. https://doi.org/10.1016/j.bbr.2008.11.020
Dickson DW (2018) Neuropathology of Parkinson disease. Parkinsonism Relat Disord 46 Suppl 1 (Suppl 1):S30-S33. https://doi.org/10.1016/j.parkreldis.2017.07.033
Sanjari Moghaddam H, Ghazi Sherbaf F, Mojtahed Zadeh M, Ashraf-Ganjouei A, Aarabi MH (2018) Association between peripheral inflammation and DATSCAN data of the striatal nuclei in different motor subtypes of Parkinson disease. Front Neurol 9:234. https://doi.org/10.3389/fneur.2018.00234
Löhle M, Wolz M, Beuthien-Baumann B, Oehme L, van den Hoff J, Kotzerke J, Reichmann H, Storch A (2020) Olfactory dysfunction correlates with putaminal dopamine turnover in early de novo Parkinson’s disease. J Neural Transm (Vienna) 127(1):9–16. https://doi.org/10.1007/s00702-019-02122-9
Oh YS, Kim JS, Hwang EJ, Lyoo CH (2018) Striatal dopamine uptake and olfactory dysfunction in patients with early Parkinson’s disease. Parkinsonism Relat Disord 56:47–51. https://doi.org/10.1016/j.parkreldis.2018.06.022
Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, Obeso J, Marek K, Litvan I, Lang AE, Halliday G, Goetz CG, Gasser T, Dubois B, Chan P, Bloem BR, Adler CH, Deuschl G (2015) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30(12):1591–1601. https://doi.org/10.1002/mds.26424
Herman T, Weiss A, Brozgol M, Giladi N, Hausdorff JM (2014) Gait and balance in Parkinson’s disease subtypes: objective measures and classification considerations. Journal of Neurology 2014 261:12 261 (12):2401–2410. https://doi.org/10.1007/S00415-014-7513-6
Stebbins GT, Goetz CG, Burn DJ, Jankovic J, Khoo TK, Tilley BC (2013) How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson’s disease rating scale: comparison with the unified Parkinson’s disease rating scale. Movement disorders : official journal of the Movement Disorder Society 28(5):668–670. https://doi.org/10.1002/mds.25383
Kang GA, Bronstein JM, Masterman DL, Redelings M, Crum JA, Ritz B (2005) Clinical characteristics in early Parkinson’s disease in a central California population-based study. Mov Disord 20(9):1133–1142. https://doi.org/10.1002/MDS.20513
Seibyl J, Jennings D, Grachev I, Coffey C, Marek K (2013) 123-I ioflupane SPECT measures of Parkinson disease progression in the Parkinson Progression Marker Initiative (PPMI) trial. J Nucl Med 54(supplement 2):190
Espay AJ, Schwarzschild MA, Tanner CM, Fernandez HH, Simon DK, Leverenz JB, Merola A, Chen-Plotkin A, Brundin P, Kauffman MA, Erro R, Kieburtz K, Woo D, Macklin EA, Standaert DG, Lang AE (2017) Biomarker-driven phenotyping in Parkinson’s disease: a translational missing link in disease-modifying clinical trials. Movement disorders : official journal of the Movement Disorder Society 32(3):319–324. https://doi.org/10.1002/mds.26913
Tang CC, Poston KL, Eckert T, Feigin A, Frucht S, Gudesblatt M, Dhawan V, Lesser M, Vonsattel J-P, Fahn S, Eidelberg D (2010) Differential diagnosis of parkinsonism: a metabolic imaging study using pattern analysis. Lancet Neurol 9(2):149–158. https://doi.org/10.1016/S1474-4422(10)70002-8
Jankovic J, Kapadia AS (2001) Functional decline in Parkinson disease. Arch Neurol 58(10):1611–1615. https://doi.org/10.1001/archneur.58.10.1611
Balint B, Mulroy E, Gövert F, Latorre A, Di Lazarro G, Erro R, Batla A, Holton JL, Miki Y, Warner TT, Bhatia KP (2021) Development of parkinsonism after long-standing cervical dystonia - a cohort. J Neurol Sci 427:117477. https://doi.org/10.1016/j.jns.2021.117477
Rudzińska M, Marona M, Bukowczan S, Banaszkiewicz K, Mirek E, Szczudlik A (2007) Falls in different types of Parkinson’s disease. Neurol Neurochir Pol 41(5):395–403
Fullard ME, Morley JF, Duda JE (2017) Olfactory dysfunction as an early biomarker in Parkinson’s disease. Neurosci Bull 33(5):515–525
Braak H, Del Tredici K, Rüb U, De Vos RA, Steur ENJ, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24(2):197–211
Hawkes CH, Del Tredici K, Braak H (2010) A timeline for Parkinson’s disease. Parkinsonism Relat Disord 16(2):79–84
Beach TG, White CL, Hladik CL, Sabbagh MN, Connor DJ, Shill HA, Sue LI, Sasse J, Bachalakuri J, Henry-Watson J (2009) Olfactory bulb α-synucleinopathy has high specificity and sensitivity for Lewy body disorders. Acta Neuropathol 117(2):169–174
Modugno N, Lena F, Di Biasio F, Cerrone G, Ruggieri S, Fornai F (2013) A clinical overview of non-motor symptoms in Parkinson’s disease. Arch Ital Biol 151(4):148–168
Oh Y-S, Kim J-S, Yoo S-W, Hwang E-J, Lyoo CH, Lee K-S (2019) Striatal dopamine activity and myocardial 123I-metaiodobenzylguanidine uptake in early Parkinson’s disease. Parkinsonism Relat Disord 63:156–161
Siderowf A, Newberg A, Chou K, Lloyd M, Colcher A, Hurtig H, Stern M, Doty R, Mozley P, Wintering N (2005) [99mTc] TRODAT-1 SPECT imaging correlates with odor identification in early Parkinson disease. Neurology 64(10):1716–1720
Berendse HW, Roos DS, Raijmakers P, Doty RL (2011) Motor and non-motor correlates of olfactory dysfunction in Parkinson’s disease. J Neurol Sci 310(1–2):21–24
Jennings D, Siderowf A, Stern M, Seibyl J, Eberly S, Oakes D, Marek K, Investigators P (2014) Imaging prodromal Parkinson disease: the Parkinson associated risk syndrome study. Neurology 83(19):1739–1746
Daalen JMJ, Tosserams A, Mahlknecht P, Seppi K, Bloem BR, Darweesh SK (2021) Towards subgroup-specific risk estimates: a meta-analysis of longitudinal studies on olfactory dysfunction and risk of Parkinson’s disease. Parkinsonism & Related Disorders
Lee JW, Song YS, Kim H, Ku BD, Lee WW (2019) Alteration of tremor dominant and postural instability gait difficulty subtypes during the progression of Parkinson’s disease: analysis of the PPMI cohort. Front Neurol 10:471. https://doi.org/10.3389/fneur.2019.00471
Nabizadeh F, Pirahesh K, Khalili E (2022) Olfactory dysfunction is associated with motor function only in tremor-dominant Parkinson’s disease. Neurol Sci. https://doi.org/10.1007/s10072-022-05952-w
Acknowledgements
PPMI—a public-private partnership—is funded by the Michael J. Fox Foundation for Parkinson’s Research funding partners 4D Pharma, Abbvie, Acurex Therapeutics, Allergan, Amathus Therapeutics, ASAP, Avid Radiopharmaceuticals, Bial Biotech, Biogen, BioLegend, Bristol-Myers Squibb, Calico, Celgene, Dacapo Brain Science, Denali, The Edmond J. Safra Foundation, GE Healthcare, Genentech, GlaxoSmithKline, Golub Capital, Handl Therapeutics, Insitro, Janssen Neuroscience, Lilly, Lundbeck, Merck, Meso Scale Discovery, Neurocrine Biosciences, Pfizer, Piramal, Prevail, Roche, Sanofi Genzyme, Servier, Takeda, Teva, UCB, Verily, and Voyager Therapeutics.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Since the data in this paper were obtained from the PPMI database (ppmi.loni.usc.edu), it does not include any research involving human or animal subjects.
Consent for publication
This manuscript has been approved for publication by all authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nabizadeh, F., Sodeifian, F. & Pirahesh, K. Olfactory dysfunction and striatal dopamine transporter binding in motor subtypes of Parkinson’s disease. Neurol Sci 43, 4745–4752 (2022). https://doi.org/10.1007/s10072-022-06110-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-022-06110-y