Abstract
Background
Molecular mechanisms underlying trigeminal neuralgia (TN) have been poorly understood. Recently, different biomarkers have been studied in several chronic neuropathic diseases or in neuronal damage, but their role in TN has not yet been investigated. Here, we firstly analyzed the serum levels of the neuron-specific enolase (NSE) (as an index of neuronal tissue damage) in TN patients submitted to surgical treatment. Different cytokines and interleukins related to inflammation were also studied.
Methods
Blood samples from 40 patients were prospectively collected preoperatively and after the surgical procedure, namely microvascular decompression (MVD) and percutaneous balloon compression (PBC). Serum levels of uric acid, NSE, ferritin, CRP, IL-2R, and IL-6 were studied. The acute pain relief (APR) and the pre- and postoperative BNI were used to evaluate the clinical outcome.
Results
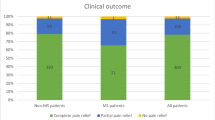
Overall, we obtained an APR in 87.5% of patients and a significant reduction of BNI after surgery (p < 0.0001). We observed a significant reduction of postoperative NSE values in the group of patients undergoing MVD (p = 0.0055) and a significant increase of postoperative NSE values in patients undergoing PBC (p < 0.05). Furthermore, in the group of patients undergoing MVD, we found a significant postoperative increase of CRP (p < 0.0001), ferritin (p = 0.001), and IL-6 (p = 0.01) values. The only patient who did not respond to MVD had NSE levels unchanged.
Conclusion
Our results suggest the hypothesis that TN would be related to the neural damage instead of the systemic inflammatory status and indicate NSE as a possible biomarker of response in patients submitted to MVD.
Similar content being viewed by others
References
de Toledo IP, Conti Réus J, Fernandes M et al (2016) Prevalence of trigeminal neuralgia: a systematic review. J Am Dent Assoc 147:570-576.e2
Rozen TD (2004) Trigeminal neuralgia and glossopharyngeal neuralgia. Neurol Clin 22:185–206
Burchiel KJ, Hodge CJ, Kanpolat Y et al (2003) A new classification for facial pain. Neurosurgery 53:1164–1167
Olesen J (2018) Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 38:1–211
Cruccu G, di Stefano G, Truini A (2020) Trigeminal neuralgia. N Engl J Med 383:754–762. https://doi.org/10.1056/NEJMra1914484
Montano N, Conforti G, di Bonaventura R et al (2015) Advances in diagnosis and treatment of trigeminal neuralgia. Ther Clin Risk Manag 11:289–299. https://doi.org/10.2147/TCRM.S37592
Sessle BJ, Hu JW (1991) Mechanisms of pain arising from articular tissues. Can J Physiol Pharmacol 69:617–26. https://doi.org/10.1139/y91-092
Dubner R, Sharav Y, Gracely RH, Price DD (1987) Idiopathic trigeminal neuralgia: sensory features and pain mechanisms Pain 31:23–33. https://doi.org/10.1016/0304-3959(87)90003-0
Jia Dze, Li G (2010) Bioresonance hypothesis: a new mechanism on the pathogenesis of trigeminal neuralgia. Med Hypotheses 74:505–507. https://doi.org/10.1016/j.mehy.2009.09.056
Devor M, Amir R, Rappaport ZH (2002) Pathophysiology of trigeminal neuralgia: the ignition hypothesis. Clin J Pain 18:4–13. https://doi.org/10.1097/00002508-200201000-00002
Latrémolière A, Mauborgne A, Masson J et al (2008) Differential implication of proinflammatory cytokine interleukin-6 in the development of cephalic versus extracephalic neuropathic pain in rats. J Neurosci 28:8489–8501. https://doi.org/10.1523/JNEUROSCI.2552-08.2008
Sommer C, Leinders M, Üçeyler N (2018) Inflammation in the pathophysiology of neuropathic pain. Pain 159:595–602. https://doi.org/10.1097/j.pain.0000000000001122
Liu MX, Zhong J, Xia L et al (2019) A correlative analysis between inflammatory cytokines and trigeminal neuralgia or hemifacial spasm. Neurol Res 41:335–340. https://doi.org/10.1080/01616412.2018.1564188
Chang B, Guan H, Zhu W, Li S (2019) Low uric acid indicates risk of incidence of trigeminal neuralgia. J Craniofac Surg 30:e556–e558. https://doi.org/10.1097/SCS.0000000000005497
Isgrò MA, Bottoni P, Scatena R (2015) Neuron-specific enolase as a biomarker: biochemical and clinical aspects. Adv Exp Med Biol 867:125–43. https://doi.org/10.1007/978-94-017-7215-0_9
Haque A, Polcyn R, Matzelle D, Banik NL (2018) New insights into the role of neuron-specific enolase in neuro-inflammation, neurodegeneration, and neuroprotection. Brain Sci 8:33. https://doi.org/10.3390/brainsci8020033
Anand N, Stead LG (2005) Neuron-specific enolase as a marker for acute ischemic stroke: a systematic review. Cerebrovasc Dis 20:213–219. https://doi.org/10.1159/000087701
Song SW, Kim YH, Kim JW et al (2018) Outcomes after transcranial and endoscopic endonasal approach for tuberculum meningiomas—a retrospective comparison. World Neurosurg 109:e434–e445. https://doi.org/10.1016/j.wneu.2017.09.202
Chabok SY, Moghadam AD, Saneei Z et al (2012) Neuron-specific enolase and S100BB as outcome predictors in severe diffuse axonal injury. J Trauma Acute Care Surg 72:1654–1657. https://doi.org/10.1097/TA.0b013e318246887e
Haque A, Ray SK, Cox A, Banik NL (2016) Neuron specific enolase: a promising therapeutic target in acute spinal cord injury. Metab Brain Dis 31:487–495
Kirino T, Brightman MW, Oertel WH, Schmechel DE, Marangos PJ (1983) Neuron-specific enolase as an index of neuronal regeneration and reinnervation. J Neurosci 3:915–23. https://doi.org/10.1523/JNEUROSCI.03-05-00915.1983
Legninda Sop FY, D’Ercole M, Izzo A et al (2021) The impact of neuronavigation on the surgical outcome of microvascular decompression for trigeminal neuralgia. World Neurosurg 149:80–85. https://doi.org/10.1016/j.wneu.2021.02.063
Montano N, Gaudino S, Giordano C et al (2019) Possible prognostic role of magnetic resonance imaging findings in patients with trigeminal neuralgia and multiple sclerosis who underwent percutaneous balloon compression: report of our series and literature review. World Neurosurg 125:e575–e581. https://doi.org/10.1016/j.wneu.2019.01.134
Cheshire WP (1997) Trigeminal neuralgia: a guide to drug choice. CNS Drugs 7:98–110. https://doi.org/10.2165/00023210-199707020-00002
Sharouf F, Hussain RN, Hettipathirannahelage S et al (2020) C-reactive protein kinetics post elective cranial surgery. A prospective observational study. Br J Neurosurg 34:46–50. https://doi.org/10.1080/02688697.2019.1680795
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The present study was approved by Fondazione IRCCS Policlinico A. Gemelli’s ethical committee and was conducted in accordance with the ethical standards reported in the 1964 Declaration of Helsinki.
Informed consent
Informed consent to the study has been provided to all participants and authorization for anonymous publication of personal data was obtained.
Conflict of interest
None.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rapisarda, A., Baroni, S., Gentili, V. et al. The role of biomarkers in drug-resistant trigeminal neuralgia: a prospective study in patients submitted to surgical treatment. Neurol Sci 43, 4425–4430 (2022). https://doi.org/10.1007/s10072-022-05971-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-022-05971-7