Abstract
Purpose
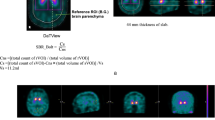
We developed a new analytical method to quantify the dopamine transporter (DAT) radiation dose in the striatum on [123I] FP-CIT single-photon emission computed tomography (SPECT). This method is based on the dopamine transporter standardized uptake value (DaTSUV). The purpose of this study was to compare DaTSUV with the classical specific binding ratio (SBR) in the discrimination of dopaminergic neurodegenerative diseases (dNDD) from non-dNDD.
Method
Seventy-seven consecutive patients who underwent DaTscan were included. Patients were divided into a dNDD group (n = 44; 24 men, 20 women; median age 73 years) and a non-dNDD group (n = 33; 14 men, 19 women; median age 75 years) based on their clinical diagnoses. The relationship between each method was evaluated by Pearson’s correlation coefficient. Differences in SBR and DaTSUV in each group were evaluated by t test. Pairwise comparison of receiver operating characteristic (ROC) curve analysis was performed to compare the discriminating abilities of each method according to the standard error of the area under the curve (AUC). A value of p < 0.05 was considered statistically significant.
Result
There was a significant strong correlation between DaTSUV and SBR (r = 0.910 [95% CI = 0.862–0.942], p < 0.001). The dNDD group showed significantly lower SBR (3.48 [1.25–7.91] vs 6.58 [3.81–11.1], p < 0.001) and DaTSUV (4.91 [1.59–13.6] vs 8.61 [2.29–15.6], p < 0.001) than the non-dNDD group. The discriminating ability of SBR (AUC = 0.918) was significantly higher than that of DaTSUV (AUC = 0.838, p = 0.0176).
Conclusion
DaTSUV has a good correlation with SBR, but it could not exceed SBR for discriminating dNDD from non-dNDD.



Similar content being viewed by others
References
Suwijn SR, van Boheemen CJ, de Haan RJ, Tissingh G, Booij J, de Bie RM (2015) The diagnostic accuracy of dopamine transporter SPECT imaging to detect nigrostriatal cell loss in patients with Parkinson’s disease or clinically uncertain parkinsonism: a systematic review. EJNMMI Res 5:12
Politis M (2014) Neuroimaging in Parkinson disease: from research setting to clinical practice. Nat Rev Neurol 10:708–722
Cummings JL, Henchcliffe C, Schaier S, Simuni T, Waxman A, Kemp P (2011) The role of dopaminergic imaging in patients with symptoms of dopaminergic system neurodegeneration. Brain J Neurol 134:3146–3166
Booth TC, Nathan M, Waldman AD, Quigley A-M, Schapira AH, Buscombe J (2015) The role of functional dopamine-transporter SPECT imaging in parkinsonian syndromes, part 1. AJNR Am J Neuroradiol 36:236–244
Booth TC, Nathan M, Waldman AD, Quigley A-M, Schapira AH, Buscombe J (2015) The role of functional dopamine-transporter SPECT imaging in parkinsonian syndromes, part 2. AJNR Am J Neuroradiol 36:236–244
Kish SJ, Shannak K, Hirnykiewicz O (1988) Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease. Pathophysiological and clinical implications. N Engl J Med 318:876–856
Asenbaum S, Pirker W, Angelberger P, Bencsits G, Pruckmayer M, Brucke T (1998) [123I] beta-CIT and SPECT in essential tremor and Parkinson’s disease. J Neural Transm 105:1213–1228
Ceravolo R, Volterrani D, Gambaccini G, Bernardini S, Rossi C, Logi C, Tognoni G, Manca G, Mariani G, Bonuccelli U, Murri L (2004) Presynaptic nigrostriatal function in a group of Alzheimer’s disease patients with parkinsonism: evidence from a dopamine transporter imaging study. J Neural Transm 111:1065–1073
Walker Z, Jaros E, Walker RW, Lee L, Costa DC, Livingston G et al (2007) Dementia with Lewy bodies: a comparison of clinical diagnosis, FP-CIT single photon emission computed tomography imaging and autopsy. J Neurol Neurosurg Psychiatry 78:1176–1181
Pirker W, Djamshidian S, Asenbaum S, Gerschlager W, Tribl G, Hoffman M et al (2002) Progression of dopaminergic degeneration in Parkinson’s disease and atypical parkinsonism: a longitudinal beta-CIT SPECT study. Mov Disord 17:45–53
Varrone A, Marek KL, Jennings D, Innís RB, Seibyl JP (2001) [123I] b-CIT SPECT imaging demonstrates reduced density of striatal dopamine transporters in Parkinson’s disease and multiple system atrophy. Mov Disord 16:1023–1032
Brücke T, Asenbaum S, Pirker W, Djamshidian S, Wenger S, Wober C et al (1997) Measurement of the dopaminergic degeneration in Parkinson’s disease with [123I] beta-CIT and SPECT. Correlation with clinical findings and comparison with multiple system atrophy and progressive supranuclear palsy. J Neural Transm Suppl 50:9–24
Benamer TS, Patterson J, Grosset DG, Booij J, de Bruin K, van Royen E et al (2000) Accurate differentiation of parkinsonism and essential tremor using visual assessment of [123I]-FP-CIT SPECT imaging: the [123I]-FP-CIT Study Group. Mov Disord 15:503–510
Schneider SA, Edwards MJ, Mir P, Cordivari C, Hooker J, Dickson J, Quinn N, Bhatia KP (2007) Patients with adult-onset dystonic tremor resembling parkinsonism tremor have scans without evidence of dopaminergic deficit (SWEDDs). Mov Disord 22(15):2210–2215
Gerschlager W, Bencsits G, Pirker W, Bloem BR, Asenbaum S, Prayer D, Zijlmans JCM, Hoffmann M, Brücke T (2002) [123I] beta-CIT SPECT distinguishes vascular parkinsonism from Parkinson’s disease. Mov Disord 17:518–523
Lorberboym M, Treves TA, Melamed E, Lampl Y, Hellman M, Djaldetti R (2006) [123I]-FP/CIT SPECT imaging for distinguishing drug-induced parkinsonism from Parkinson’s disease. Mov Disord 21(4):510–514
Benaderette S, Zanotti Fregonara P, Apartis E, Nguyen C, Trocello JM, Remy P et al (2006) Psychogenic parkinsonism: a combination of clinical, electrophysiological, and [123I]-FP-CIT SPECT scan explorations improves diagnostic accuracy. Mov Disord 21:310–317
Colloby SJ, Firbank MJ, Pakrasi S, Lloyd JJ, Driver I, McKeith IG et al (2008) A comparison of 99mTc-exametazime and 123I-FP-CIT-SPECT imaging in the differential diagnosis of Alzheimer’s disease and dementia with Lewy bodies. Int Psychogeriatr 20:1124–1140
Nicastro N, Garibotto V, Allali G, Assal F, Burkhard PR (2017) Added value of combined semi-quantitative and visual [123I] FP-CIT SPECT analyses for the diagnosis of dementia with Lewy bodies. Clin Nucl Med 42:96–102
Tossici Bolt L, Hoffmann SM, Kemp PM, Mehta RL, Fleming JS (2006) Quantification of [123I] FP-CIT SPECT brain images: an accurate technique for measurement of the specific binding ratio. Eur J Nucl Med Mol Imaging 33:1491–1499
Bailey DL, Willowson KP (2013) An evidence-based review of quantitative SPECT imaging and potential clinical applications. J Nucl Med 54:83–89
Ritt P, Vija H, Hornegger J, Kuwert T (2011) Absolute quantification in SPECT. Eur J Nucl Med Mol Imaging 38:69–77
Welz F, Sanders JC, Kuwert T, Maler J, Kornhuber J, Ritt P (2016) Absolute SPECT/CT quantification of cerebral uptake of 99mTc-HMPAO for patients with neurocognitive disorders. Nuklearmedizin 55:158–165
Shrestha U, Sciammarella M, Alhassen F, Yeghiazarians Y, Ellin J, Verdin E, Boyle A, Seo Y, Botvinick EH, Gullberg GT (2017) Measurement of absolute myocardial blood flow in humans using dynamic cardiac SPECT and 99mTc-tetrofosmin: method and validation. J Nucl Cardiol 24:268–277
Cachovan M, Vija AH, Hornegger J, Kuwert T (2013) Quantification of 99mTc-DPD concentration in the lumbar spine with SPECT/CT. EJNMMI Res 5:45
Beck M, Sanders JC, Ritt P, Reinfelder J, Kuwert T (2016) Longitudinal analysis of bone metabolism using SPECT/CT and (99m) Tc-diphosphono-propanedicarboxylic acid: comparison of visual and quantitative analysis. EJNMMI Res 6:60
Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, Obeso J, Marek K, Litvan I, Lang AE, Halliday G, Goetz CG, Gasser T, Dubois B, Chan P, Bloem BR, Adler CH, Deuschl G (2015) MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord 30:1591–1601
Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, Wood NW, Colosimo C, Durr A, Fowler CJ, Kaufmann H, Klockgether T, Lees A, Poewe W, Quinn N, Revesz T, Robertson D, Sandroni P, Seppi K, Vidailhet M (2008) Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71:670–676
Höglinger GU, Respondek G, Stamelou M, Kurz C, Josephs KA, Lang AE, Mollenhauer B, Müller U, Nilsson C, Whitwell JL, Arzberger T, Englund E, Gelpi E, Giese A, Irwin DJ, Meissner WG, Pantelyat A, Rajput A, van Swieten JC, Troakes C, Antonini A, Bhatia KP, Bordelon Y, Compta Y, Corvol JC, Colosimo C, Dickson DW, Dodel R, Ferguson L, Grossman M, Kassubek J, Krismer F, Levin J, Lorenzl S, Morris HR, Nestor P, Oertel WH, Poewe W, Rabinovici G, Rowe JB, Schellenberg GD, Seppi K, van Eimeren T, Wenning GK, Boxer AL, Golbe LI, Litvan I, for the Movement Disorder Society-endorsed PSP Study Group (2017) Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord 32:853–864
McKeith IG, Boeve BF, Dickson DW et al (2017) Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology 89:88–100
Armstrong MJ, Litvan I, Lang AE, Bak TH, Bhatia KP, Borroni B, Boxer AL, Dickson DW, Grossman M, Hallett M, Josephs KA, Kertesz A, Lee SE, Miller BL, Reich SG, Riley DE, Tolosa E, Troster AI, Vidailhet M, Weiner WJ (2013) Criteria for the diagnosis of corticobasal degeneration. Neurology 80:496–503
Zeint J, Vija AH, Yahil A, Hornegger J, Kuwert T (2010) Quantitative accuracy of clinical 99mTc SPECT/CT using ordered-subset expectation maximization with 3-dimensional resolution recovery, attenuation, and scatter correction. J Nucl Med 51:921–928
Thie JA (2004) Understanding the standardized uptake value, its methods, and implications for usage. J Nucl Med 45:1431–1434
Marshall VL, Reininger CB, Marquardt M, Patterson J, Hadley DM, Oertel WH, Benamer HTS, Kemp P, Burn D, Tolosa E, Kulisevsky J, Cunha L, Costa D, Booij J, Tatsch K, Chaudhuri KR, Ulm G, Pogarell O, Höffken H, Gerstner A, Grosset DG (2009) Parkinson’s disease is overdiagnosed clinically at baseline in diagnostically uncertain cases: a 3-year European multicenter study with repeat [123I] FP-CIT SPECT. Mov Disord 24:500–508
Ueda J, Yoshimura H, Shimizu K, Hino M, Kohara N (2017) Combined visual and semi-quantitative assessment of 123I-FP-CIT SPECT for the diagnosis of dopaminergic neurodegenerative diseases. Neurol Sci 38:1187–1191
Shimizu S, Namioka N, Hirose D et al (2017) Comparison of diagnostic utility of semi-quantitative analysis for DAT-SPECT for distinguishing DLB from AD. J Neurol Sci 377:50–54
Yokoyama K, Imabayashi E, Sumida K et al (2017) Computed-tomography-guided anatomic standardization for quantitative assessment of dopamine transporter SPECT. Eur J Nucl Med Mol Imaging 44:366–372
Varrone A, Dickson JC, Tossici-Bolt L, Sera T, Asenbaum S, Booij J, Kapucu OL, Kluge A, Knudsen GM, Koulibaly PM, Nobili F, Pagani M, Sabri O, Vander Borght T, van Laere K, Tatsch K (2013) European multicentre database of healthy controls for [123I] FP-CIT SPECT (ENC-DAT): age-related effects, gender differences and evaluation of different methods of analysis. Eur J Nucl Med Mol Imaging 40:213–227
Abi-Dargham A, Innis RB, Wisniewski G, Baldwin RM, Neumeyer JL, Seibyl JP (1997) Human biodistribution and dosimetry of iodine-123-fluoroalkyl analogs of beta-CIT. Eur J Nucl Med 24:1422–1425
Booij J, Busemann Sokole E, Stabin MG, Janssen AG, de Bruin K, van Royen EA (1998) Human biodistribution and dosimetry of [123I] FP-CIT: a potent radioligand for imaging of dopamine transporters. Eur J Nucl Med 25:24–30
Acknowledgements
We thank Takashi Okunaga and Kazuhiro Kubo, the technologists at Kobe University Graduate School of Medicine, for their help during system quality control and acquisition of the data in patient examinations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wakabayashi, Y., Takahashi, R., Kanda, T. et al. Semi-quantitative dopamine transporter standardized uptake value in comparison with conventional specific binding ratio in [123I] FP-CIT single-photon emission computed tomography (DaTscan). Neurol Sci 39, 1401–1407 (2018). https://doi.org/10.1007/s10072-018-3437-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-018-3437-8