Abstract
This document presents the guidelines for onconeural antibody testing that have been developed following a consensus process built on questionnaire-based surveys, internet contacts, and discussions at workshops of the sponsoring Italian Association of Neuroimmunology (AINI) congresses. Essential clinical information on paraneoplastic neurological syndromes, indications and limits of onconeural antibody testing, instructions for result interpretation, and an agreed laboratory protocol (Appendix) are reported for the communicative community of neurologists and clinical pathologists.

Similar content being viewed by others
References
Moll JW, Antoine JC, Brashear HR et al (1995) Guidelines on the detection of paraneoplastic anti-neuronal-specific antibodies: report from the workshop to the fourth meeting of the International Society of Neuro-Immunology on paraneoplastic neurological disease, October 22-23, 1994, Rotterdam, The Netherlands. Neurology 45:1937–1941
Graus F, Delattre JY, Antoine JC et al (2004) Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry 75:1135–1140
Giometto B, Grisold W, Vitaliani R, Graus F, Honnorat J, Bertolini G, Euronetwork PNS (2010) Paraneoplastic neurologic syndrome in the PNS Euronetwork database: a European study from 20 centers. Arch Neurol 67:330–335
Darnell RB, Posner JB (2003) Paraneoplastic syndromes involving the nervous system. N Engl J Med 349:1543–1554
Graus F, Cordon-Cardo C, Posner JB (1985) Neuronal antinuclear antibody in sensory neuronopathy from lung cancer. Neurology 35:538–543
Darnell RB (2004) Paraneoplastic neurologic disorders: windows into neuronal function and tumor immunity. Arch Neurol 61:30–32
Monstad SE, Knudsen A, Salvesen HB, Aarseth JH, Vedeler CA (2009) Onconeural antibodies in sera from patients with various types of tumours. Cancer Immunol Immunother 58:1795–1800
Dalmau J, Graus F, Cheung N et al (1995) Major histocompatibility proteins, anti-Hu antibodies, and paraneoplastic encephalomyelitis in neuroblastoma and small cell lung cancer. Cancer 75:99–109
Maverakis E, Goodarzi H, Wehrli LN, Ono Y, Garcia MS (2012) The etiology of paraneoplastic autoimmunity. Clin Rev Allergy Immunol 42:135–144
Storstein A, Raspotnig M, Vitaliani R et al (2016) Prostate cancer, Hu antibodies and paraneoplastic neurological syndromes. J Neurol 263:1001–1007
Titulaer MJ, Soffietti R, Dalmau J et al (2011) Screening for tumours in paraneoplastic syndromes: report of an EFNS task force. Eur J Neurol 18:19–e3
Lladó A, Carpentier AF, Honnorat J et al (2006) Hu-antibody-positive patients with or without cancer have similar clinical profiles. J Neurol Neurosurg Psychiatry 77:996–997
Ducray F, Demarquay G, Graus F et al (2014) Seronegative paraneoplastic cerebellar degeneration: the PNS Euronetwork experience. Eur J Neurol 21:731–735
Ducray F, Graus F, Vigliani MC et al (2010) Delayed onset of a second paraneoplastic neurological syndrome in eight patients. J Neurol Neurosurg Psychiatry 81:937–939
Psimaras D, Carpentier AF, Rossi C, Euronetwork PNS (2010) Cerebrospinal fluid study in paraneoplastic syndromes. J Neurol Neurosurg Psychiatry 81:42–45
Vedeler CA, Antoine JC, Giometto B et al (2006) Management of paraneoplastic neurological syndromes: report of an EFNS task force. Eur J Neurol 13:682–690
Grisold W, Giometto B, Vitaliani R, Oberndorfer S (2011) Current approaches to the treatment of paraneoplastic encephalitis. Ther Adv Neurol Disord 4:237–248
Giometto B, Vitaliani R, Lindeck-Pozza E, Grisold W, Vedeler C (2012) Treatment for paraneoplastic neuropathies. Cochrane Database Syst Rev 12:CD007625
Briani C, Vitaliani R, Grisold W et al (2011) Spectrum of paraneoplastic disease associated with lymphoma. Neurology 76:705–710
Titulaer MJ, Maddison P, Sont JK et al (2011) Clinical Dutch–English Lambert–Eaton myasthenic syndrome (LEMS) tumor association prediction score accurately predicts small-cell lung cancer in the LEMS. J Clin Oncol 29:902–908
Graus F, Dalmau J (2012) Paraneoplastic neurological syndrome. Curr Opin Neurol 25:795–801
Molinuevo JL, Graus F, Serrano C et al (1998) Utility of anti-Hu antibodies in the diagnosis of paraneoplastic sensory neuropathy. Ann Neurol 44:976–980
Moll JW, Henzen-Logmans SC, Splinter TA, van der Burg ME, Vecht CJ (1990) Diagnostic value of anti-neuronal antibodies for paraneoplastic disorders of the nervous system. J Neurol Neurosurg Psychiatry 53:940–943
de Graaff E, Maat P, Hulsenboom E et al (2012) Identification of delta/notch-like epidermal growth factor-related receptor as the Tr antigen in paraneoplastic cerebellar degeneration. Ann Neurol 71:815–824
Probst C, Komorowski L, de Graaff E et al (2015) Standardized test for anti-Tr/DNER in patients with paraneoplastic cerebellar degeneration. Neurol Neuroimmunol Neuroinflamm 2:e68
Gorr TA, Vogel J (2015) Western blotting revisited: critical perusal of underappreciated technical issues. Proteomics Clin Appl 9:396–405
Sabater L, Saiz A, Dalmau J, Graus F (2016) Pitfalls in the detection of CV2 (CRMP5) antibodies. J Neuroimmunol 290:80–83
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest
Appendix
Appendix
-
1.0
Preanalytical procedures
Refer to the document on “Diagnostics of autoimmune encephalitis associated with antibodies against neuronal surface antigens”
-
2.0
Analytical procedures
-
2.1
Indirect immunofluorescence. Procedures refer to commercial BIOCHIP Slides by Euroimmun (Lübeck, Germany).
-
2.1.1
Reagent preparation
-
2.1.1.1
BIOCHIP Slides with monkey cerebellum are ready for use and sealed in a package that must be opened when room temperature has been reached (18–25 °C) to prevent condensation. Do not touch the biochip.
-
2.1.1.2
Fluoresceinated goat anti-human Ig antibody (mix thoroughly before use).
-
2.1.1.3
Mounting medium.
-
2.1.1.4
Negative and positive control sera.
-
2.1.1.5
PBS-Tween Wash Solution: dissolve 1 package of “phosphate buffer salt” in 1 l of high-grade distilled water, add 2 mL of Tween 20, and stir for at least 20 min; store in a refrigerator (2–8 °C) for 1 week.
-
2.1.1.6
Glassy reagent supports: thorough rinses with water and accurate drying are recommended after washing (see the instruction manual).
-
2.1.1.1
-
2.1.2
Sample preparation
-
2.1.2.1
Vortex the samples after thawing. Dilute serum samples 1:10 in PBS-Tween 20 (11 μL of sample in 100 μL of PBS-Tween 20) and mix thoroughly (vortex). CSF samples are tested undiluted.
-
2.1.2.2
For titration, go on the 1:10 dilution (1:100, 1:1000, 1:10,000, up to the end-point titration).
-
2.1.2.1
-
2.1.3
Analytical procedure
-
2.1.3.1
Dispense 25 μL of each diluted serum or undiluted CSF sample on the well of the glassy tray, preventing the formation of air bubbles, and using the polystyrene frame as a reference.
-
2.1.3.2
Dispense all the samples before starting the incubation.
-
2.1.3.3
Start the incubation after putting the transfected-cell-equipped biochips onto the glassy tray, checking that each sample is in contact with the biochip, and preventing between-sample cross-contaminations; incubate 30 min at room temperature (RT; 18–25 °C).
-
2.1.3.4
Washing: Immerse BIOCHIP Slides into a beaker containing PBS-Tween 20 and then immerse them in the appropriate PBS-Tween 20 containing cuvette for 5 min (if available, gently shake with a rotary stirrer). Be careful not to completely dry the slides (to prevent faded patterns with or without high background).
-
2.1.3.5
Dispense 25 μL of conjugated antibody to each well of the clean glassy tray.
-
2.1.3.6
Remove BIOCHIP Slides from the washing cuvettes, rapidly dry the back and sides of the slides with absorbent paper, and then place then in the appropriate glassy tray areas. Check the contact between biochips and antibody. Incubate for 30 min at RT.
-
2.1.3.7
Repeat washing (point 2.1.3.4), using fresh PBS-Tween 20.
-
2.1.3.8
Mount BIOCHIP Slides, placing the cover slides on the polystyrene supports and dispensing a drop of mounting medium (10 μL/well). Check the perfect engagement between biochip slides and cover slides.
-
2.1.3.1
-
2.1.4
Reading and interpretation of results
-
2.1.1
-
2.1
Use fluorescence microscopes (excitation filter, 450–490 nm; color separator, 510 nm; blocking filter, 515 nm) with 20×-40× magnifications. Slides are interpreted blindly by two expert observers and defined as “Positive” or “Negative”; when the interpretations are discordant, repeat the test.
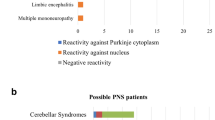
Describe how fluorescence distributes in the cerebellar tissue at various levels: neuropil, cell layers, myelinated fibers, nuclei, cytoplasm (pictures at www.euroimmun.com). As for the most common reactivities, anti-Yo antibodies stain exclusively the cytoplasm of Purkinje cells, anti-Hu and anti-Ri antibodies neuronal nuclei in the gray matter with granular fluorescence. AINI network of laboratories is available to evaluate and retest dubious samples.
-
2.2
Dot/line blot. Commercial kits are available for confirming the presence of the main onconeural autoantibodies. Recombinant proteins are fixed onto nitrocellulose stripes. Human IgG is the internal control antigen. Neuronal antigens are typically purified by affinity, separated with SDS-PAGE, and immobilized onto nitrocellulose. Autoantibodies are detected with an indirect immunoenzymatic reaction. Procedure: follow the manufacturer’s instructions.
-
2.3
Western blot. In-house Western blot of cerebellum tissue homogenates can be used as an alternative to commercial dot/line blot blots. The rational use of in-house Western blot can be restricted to the analysis of serum samples resulted positive on the immunohistochemistry screening and negative on commercial dot/line blot blots (if ANA negative).
-
3.0
QUALITY CONTROL AND SAMPLE STORAGE
-
3.1
Indirect immunofluorescence
-
3.1.1
In every analytical run a positive and a negative control should be used.
-
3.1.2
If the positive control gives no/dubious staining, and the negative control non-specific or high-background staining, repeat the test.
-
3.1.3
External quality control schemes should be performed at least yearly (e.g., AINI external quality control schemes).
-
3.1.1
-
3.2
Dot/line blot
-
3.2.1
In every analytical run a positive control should be included.
-
3.2.2
External quality control schemes should be performed at least yearly (e.g., AINI external quality control schemes).
-
3.2.1
-
3.3
Storage, see the document on ‘Cerebrospinal fluid analysis and the determination of oligoclonal bands’
-
3.1
-
4.0
REPORT
-
4.1
Indirect immunofluorescence. A qualitative result (positive/negative) should be reported, together with a detailed description of the staining localization (see point 2.1.3). Titering is optional, but advisable.
-
4.2
Dot/line blot. A qualitative result (positive/negative) should be reported, together with the indication of the type of reactivity (e.g., anti-Hu antibodies).
-
4.3
Reports should contain the following general information:
-
i)
Type of method: indirect immunofluorescence, type of tissue and name of the manufacturer.
-
ii)
Type of method: dot/line blot, name of the manufacturer.
-
iii)
Reference values: report the dilution as cut-off of positivity.
-
iv)
Comments: Refer to point 4.1.9 of the document on “Cerebrospinal fluid analysis and the determination of oligoclonal bands.”
-
i)
-
4.1
Rights and permissions
About this article
Cite this article
Zoccarato, M., Gastaldi, M., Zuliani, L. et al. Diagnostics of paraneoplastic neurological syndromes. Neurol Sci 38 (Suppl 2), 237–242 (2017). https://doi.org/10.1007/s10072-017-3031-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-017-3031-5