Abstract
Bonobos appear to show little evidence of learning to make one response (R1) to an AB sequence and a different response (R2) to sequences BB, AA, and BA (Lind et al. PLoS ONE 18(9):e0290546, 2023), yet under different conditions, pigeons can learn this (Weisman et al. Exp Psychol Anim Behav Process 6(4):312, 1980). Aspects of the bonobo procedure may have contributed to this failure. Most important, no response was required in the presence of the stimuli to encourage attention to them. Furthermore, learning to make one response to the target sequence and another to the other sequences involves a bias that allows for better than chance responding. With the two-alternative forced-choice procedure used with the bonobos, the R1 response is correct for one sequence, whereas the R2 response is correct for three sequences. To correct for this, there are three times as many AB trials as each of the other sequences. However, this correction allows a bias to develop in which reinforcement often can be obtained by using only the last stimulus seen as the basis of choice (e.g., when the last stimulus is B respond R1 when the last stimulus is A respond R2). This solution yields reinforcement on five out of six, or 83%, of the trials. In the present experiment with pigeons, using this two-alternative forced choice procedure, most subjects tended to base their choice on the last-seen stimulus. This design allowed subjects to use a suboptimal but relatively effective choice strategy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The ability of animals to make a discrete response to a sequence of two stimuli (e.g., A followed by B) and make a different discrete response to other sequences involving the same two stimuli (i.e., AA, BB, and BA) has been studied in several species (for a review, see Ghirlanda et al. 2017). Ghirlanda et al. concluded “that available evidence supports the hypothesis that non-human animals do not have the ability to faithfully recognize and represent arbitrary stimuli that are separated in time” (p. 2).
Lind et al. (2023) has shown that humans have no difficulty learning a task involving such a sequence. According to Ghirlanda et al. (2017), the difference in sequence learning between humans and other animals has important implications. They argue that the ability to learn sequences has allowed for the development of language (Ullman 2004), memories for the sequence of personal experiences (Tulving 2002), problem solving (Hambrick et al. 2020), and planning for the future (Atance & O’Neill, 2001).
The proposition that nonhuman animals cannot acquire such a sequence-learning task seems questionable. According to James (1899, p. 561), the mechanism responsible for memory for two long-remembered objects in their prior order of occurrence is none other than the law of association by contiguity. Similarly, Skinner (1950) suggested that pigeons should be able to learn to respond to green [B] after having been stimulated by red [A] just as easily as learning to respond to red [B] after having been stimulated by green [A]. Thus, one might expect that non-human animals would also be able to respond with one response to a specific stimulus sequence and with a different response to other sequences of those stimuli.
The difficulty in learning a two-stimulus sequence may be that it involves remembering the identity of a stimulus after having experienced a second stimulus and that is likely to produce retroactive interference (e.g., Zentall 1973). At the same time, this procedure requires remembering the identity of the second stimulus to determine if the two stimuli together represent the target sequence. This difficulty is well described by Christiansen and Chater (2016) in describing the importance of stimulus order for humans in decoding meaning in language. For example, word order is critical in understanding the difference in meaning between “the dog bit the girl” and “the girl bit the dog.”
As evidence of the limited ability of animals to remember the difference between the order of two stimuli, Lind et al. (2023) tested bonobo apes on a task in which a sequence of two stimuli AB (e.g., yellow followed by blue lights) required a particular response (e.g., pressing a button on the left) for reinforcement, whereas presentation of all other sequences of those two stimuli (i.e., AA, BA, and BB), required a different response (i.e., pressing a button on the right). Given that there was only one sequence that required a response on the left, whereas there were three sequences that required a response on the right, a bias to make the second response would likely develop. To control for such a possible response bias, Lind et al. included three times as many AB trials as AA, BA, or BB trials, such that there was an equal number of the two correct responses in each block of trials. Surprisingly, however, Lind et al. found little evidence that the bonobos learned to respond appropriately to the sequences. After over 2,000 trials on this task the bonobos’ accuracy was close to chance level.
This result is surprising because many years earlier, Weisman et al. (1980) found evidence that pigeons could learn a similar task. The main difference between the procedure used in the two studies appeared to be that the Lind et al. (2023) study involved a two-alternative forced-choice (left-right) discrimination, whereas the Weisman et al. (1980) study involved a go/no-go discrimination (i.e., pecking to a central stimulus was reinforced following the AB sequence but not following the AA, BA, and BB sequences). The measure of learning in the Weisman et al. study was a discrimination ratio (pecks relative to pecks to the AB sequence) and the pigeons’ accuracy was better than 0.80.
It seems unlikely that the pigeons were capable of learning the two-stimulus sequence, whereas the bonobos were not. Furthermore, it seems unlikely that the sequence discrimination can be learned only if the discrimination involves a go/no-go procedure. Alternatively, it may be that the problem that the bonobos had in the Lind et al. (2023) experiment was that they did not attend sufficiently to the stimuli. In that study, no effort was made to encourage the subjects to attend to the sequence presentation. In the Weisman et al. (1980) study, the pigeons were exposed to each of the A and B stimuli for 5 s, whereas in the Lind et al. study the bonobos were shown the two stimuli for only 1 s each.
Furthermore, in a typical two-alternative forced-choice procedure (e.g., a typical conditional discrimination) with only a single stimulus as the conditional stimulus, the only pattern of responding that provides more than 50% reinforcement is the pattern identified by the experimenter (e.g., if the sample is A choose X, if the sample is B choose Y). In the sequence learning design used by Lind et al. (2023), however, even controlling for the frequency of left and right correct choices, if an animal based its responses solely on the last stimulus seen (a recency effect), it could be correct 83% of the time. Specifically, if the animal chooses R1 when the last stimulus in the sequence is B and R2 when the last stimulus in the sequence is A, it would be correct on the three AB trials, as well as sequences AA and BA. The only sequence for which it would be incorrect would be BB. Assuming three times as many AB trials as each of the BB, AA, and BA trials, such a response pattern would result in the animal being correct on five out of six or 83% of the trials.
Although not is likely, it is also possible that an animal could base its responses solely on the first stimulus seen (a primacy effect). If the first stimulus is A, it might respond R1, but if the first stimulus is B it would respond R2. In this case, the animal would be correct on all of the AB sequence trials (R1) as well as the BB and BA sequences (R2). The only sequence for which it would be incorrect would be AA. Again, it would be correct 83% of the time. Finally, an animal could sort the sequences according to whether there was a change in the color between the first and the second stimulus (i.e., AB and BA, vs. AA and BB). In this case, the animal would be correct on all of the AB sequence trials (R1) as well as the AA and BB sequence trials (R2), and the only trials for which it would be incorrect would be the BA trials. Again, it would be correct 83% of the time. Of these three possibilities, given animals generally poor short-term memory (e.g., Roberts 1972), it is likely that they would base their choice on the last stimulus seen but any of the three hypotheses would result in better than chance accuracy.
Thus, a problem with the two-alternative forced-choice procedure when used to evaluate this kind of sequence learning in animals, is whether learning to isolate the correct sequence from the other sequences is discriminable in terms of the conditions of reinforcement from learning a simpler rule. If such a rule is easier to learn than learning to isolate the target AB sequence, the greater than 50% probability of reinforcement may be sufficient to maintain that bias.
The purpose of the present experiment was to determine if pigeons can acquire a two-item sequence discrimination using a two-alternative discrimination similar to the procedure used by Lind et al. (2023).
Method
Subjects
The subjects in this experiment were 12 White Carneau pigeons 8–12 years old, obtained from the Palmetto Pigeon Plant (Sumter, SC). The pigeons were of undetermined sex. They had previously served in a simple successive color discrimination involving the colors red, green, blue, and yellow. Early in the experiment one pigeon became ill and was dropped from the study. Throughout the experiment the pigeons were kept at 85% of their free feeding weight. They were individually housed in wire cages that measured 28 cm x 38 cm x 30.5 cm. Throughout the study, the pigeons were maintained at 85% of their free-feeding body weight with free access to water and grit. The room in which the pigeons were housed was on a 12:12-hr light: dark cycle.
Apparatus
The experiment was conducted in a BRS/LVE (Laurel, MD) sound attenuating operant test chamber measuring 34 cm high, 30 cm wide, and 35 cm across the response panel. There were three horizontally aligned round (2.5 cm diameter) response keys mounted on the panel 25 cm above the floor of the response panel and spaced 6.0 cm apart. Behind each key was a 12-stimulus in-line projector (Industrial Electronics Engineering, Van Nuys, CA). The center key could project red or green stimuli (Kodak Wratten Filters Nos. 26 or 38, respectively) while the left and right response keys could project white (unfiltered). The bottom of the center-mounted feeder was 9.5 cm from the floor. Reinforcement consisted of 2.0 s access to Brown’s Premium Pigeon Feeds. A microcomputer in an adjacent room controlled the experiment.
Procedure
As the pigeons had served in a previous experiment involving color discriminations, there was no pretraining required. Each trial of the experiment began with the illumination of the center response key, either red or green. To encourage the pigeons to attend to the stimulus presented on the lit key, five pecks were required to the lit key to turn it off for 0.5 s and then illuminate it again, either red or green. Five pecks were required to the second stimulus to turn it off and turn on white stimuli on the left and right side keys. For six of the pigeons, the target sequence was red followed by green (red-green). For three of those pigeons, if the target sequence had been presented, a peck to the left response key was reinforced. For the remaining three pigeons for which the target sequence was red-green a peck to the right response key was reinforced. For these six pigeons, if the sequence was not the target sequence (i.e., green-green, red-red, or green-red), a peck to the other response key was reinforced.
For the remaining five pigeons, the target sequence was green followed by red (green-red) with a peck to the left response key reinforced for three pigeons and a peck to the right response key reinforced for the remaining two pigeons. For these five pigeons if the sequence was not the target sequence (i.e., green-green, red-red, or red- green), a peck to the other response key was reinforced.
To avoid a bias to respond to the non-target sequence response key, for both groups, each block of 24 trials involved three times as many target sequence trials as each of the non-target sequence trials. Thus, there was an equal number of possible reinforced trials on the left and right keys. Trials were randomly selected from a list of 24 trials. Each of 40 sessions of noncorrection training consisted of three blocks of 24 trials. Sessions were conducted 6 days a week.
Although Lind et al. (2023) used a correction procedure but did not find that the bonobos learned to respond selectively to the AB sequence, a correction procedure should have the potential to breakup position biases, biases that may inhibit learning. Thus, following noncorrection training, the pigeons were placed on a modified correction procedure. During correction training, if an incorrect choice response was made, the trial was repeated to a maximum of 5 repeats. After the 5th repeat, the program moved on to the next trial. Responses on correction trials were not counted in calculating the percentage correct. Each of 30 sessions of correction training consisted of three blocks of 24 trials. Sessions were conducted 6 days a week.
Analyses
An initial analysis was conducted on the percentage correct scores using a repeated-measures one-way analysis of variance with sessions as the independent variable. Acquisition of each of the trial types was also examined to determine if the pigeons were developing a bias of the kind mentioned in the introduction. In addition, for each of the pigeons, the data were pooled over the last 5 sessions of noncorrection training and the last 5 sessions of correction training for each of the four trial types (the target sequence, and each of the non-target sequences).
Results
Acquisition
Eight of the 11 pigeons showed evidence of learning to a level better than chance. Two of the remaining three pigeons developed strong position preferences and one pigeon’s pattern of responding was difficult to classify. During noncorrection training six of the pigeons showed a discrimination based on the last stimulus seen, four showed a position preference, and one pigeon (716) had a position preference during noncorrection training but its pattern of responding during correction training was difficult to classify. During correction training, one pigeon appeared to learn the sequence (2797), one pigeon shifted from spatial to the last stimulus seen (278), and one pigeon continued to choose based on the first stimulus seen (154). Table 1 provides a summary of the primary strategy used by each pigeon during noncorrection and correction training. The classification of strategy was based the pigeon’s pattern of choices: Last (choice based on the last stimulus seen) = Accuracy on AB, AA, and BA trials above chance and accuracy on BB trials below chance. Spatial (choice all left or all right, independent of the stimulus) = Accuracy on the AB trials below chance and accuracy on BB, AA, and BA above chance (or the reverse).
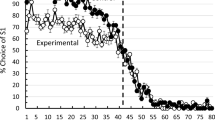
The mean acquisition function over all 40 sessions of noncorrection training is presented in Fig. 1. A Shapiro-Wilk test of normality performed on the acquisition data indicated that the data were within the normal range, p = .80. A one-way repeated-measures analysis of variance performed on the percentage correct data indicated that there was a significant effect of sessions, F(39,690) = 5.80, p < .001, n2 = .0.34. The linear trend of sessions was also statistically significant, F(1,690) = 13.58, p = .004, n2 = .028
Because accuracy on the different trial types varied greatly, acquisition functions for each of the four trial types (AB, BB, AA, and BA) are presented in Fig. 2. Initially, with the noncorrection procedure, the pigeons chose the spatial location associated with correct responding to sequences BB, AA, and BA and paradoxically, they avoided the location associated with sequence AB. With continued training, they developed a tendency to choose based on the last stimulus seen. Thus, thereafter, accuracy went up on AB sequence trials and down on BB sequence trials. During correction training the tendency to choose based on the last stimulus seen became stronger.
As can be seen in Fig. 2, accuracy on AA and BA trials during noncorrection training increased relatively systematically as a function of training, reaching accuracy close to 90% correct. When correction training was introduced, however, accuracy on AA and BA trials fell somewhat. Over the last 5 sessions of correction training, accuracy on AA trials (67.1%) was significantly above chance, t(10) = 2.20, p = .05, as was accuracy on BA trials (77.1%), t(10) = 4.77, p = .0007. Accuracy on BB trials initially increased with AA and BA trials but then decreased to near chance toward the end of noncorrection training and decreased still further with correction training. Over the last 5 sessions of correction training accuracy on BB trials (41.4%) was significantly below chance, t(10) = 3.05, p = .012. Accuracy on AB trials fell over the first seven sessions of noncorrection training and then gradually increased to above chance. Accuracy on AB trials continued to increase with correction training and over the last 5 sessions of correction training accuracy on AB trials (66.5%) was significantly above chance, t(10) = 3.43, p = .006, d = 2.17. At the end of noncorrection training, choice based on the last stimulus seen began to emerge and fully developed by the end of correction training. Over the last 5 sessions of correction training, accuracy when the last stimulus was A was quite high (72.1%), whereas accuracy when the last stimulus seen was B was barely above chance (53.9%). Of course, when the last stimulus was B, choice on AB trials was generally correct, whereas choice on BB trials was generally incorrect. In general, choice was independent of the first stimulus seen.
Individual differences
For each of the pigeons, a bar graph representing accuracy on the four trial types, pooled over the last 5 sessions of noncorrection training, and last 5 sessions of correction training, is presented in Fig. 3. From the bar graphs one can see individual differences in the way the pigeons approached learning this task. In Fig. 3, the light bars represent the last 5 sessions of noncorrection training; the dark bars represent the last 5 sessions of correction training. To better illustrate the pigeons’ basis for the sequence discrimination, bars that go downward represent accuracy below 50%.
As one can see from Fig. 3, seven of the pigeons (2797, 1016, 84, 19845, 23644, 22642, 278) showed at least some tendency during noncorrection or correction training to discriminate the trial types based primarily on the last stimulus seen. That is, they tended to make one response when the last stimulus was A and the other response when the last stimulus was B. This appears in Fig. 3 as below chance choice on BB trials. Pigeon 2797 chose based on the last stimulus seen during noncorrection training but during correction training this pigeon actually appeared to learn to respond correctly to the sequences. Pigeon 19845 showed some tendency to learn the sequence during noncorrection training but during correction training reverted to a discrimination based on the last stimulus seen.
Two pigeons had a strong position bias during noncorrection training (12295, and 26366; 67%, and 62%, respectively). Pigeon 12295 reversed that position preference during correction training. Pigeon 26366 showed some evidence of sequence learning during correction training but it was mostly learning the BA sequence at the expense of the BB and AA sequences. Pigeon 154 showed an unusual pattern of choice. It was the only pigeon that showed a tendency to use the first stimulus seen as the basis for the discrimination (below chance choice on AA trials alone). The remaining pigeon (716) responded somewhat below chance on the AB sequence (29%) and near chance on the other three sequences (45%, 48%, and 43%).
Overall, accuracy pooled over the final 5 sessions of correction training (64.2% correct) indicated that there was significant learning relative to chance (50% correct), t(10) = 3.42, p = .007, d = 2.16. A Shapiro-Wilk-test of normality performed on the individual trial types indicated that the data for all of the trial types were within the normal range, p = .66, p = .42, p = .47, and p = .11, for the AB, BB, AA, and BA trial types, respectively. To get a better feel for the difference in accuracy of the pigeons among the trial types at the end of correction training, a one-way repeated-measures analysis of variance was performed on the trial type accuracy data from each pigeon pooled over the last 5 sessions of correction training. The analysis indicated that there was a significant effect of trial type, F(3.30) = 6.63, p = .001, n2 = 0.552.
Choice pattern
Although we originally proposed that there were three possible suboptimal rules the pigeons might use (last stimulus seen, first stimulus seen, or stimulus change), given pigeons’ relatively poor short-term memory, we suspected that the last stimulus seen was the one most likely to be used. If so, a pigeon would likely sort the AB and BB sequences together (B seen last) and the AA and BA sequences together (A seen last). Thus, it would be predicted that accuracy on the BB sequence would be poorest because it would be the only sequence to which the pigeon would respond incorrectly. To determine if accuracy on the BB trial type (41.4% correct) was different from the other three trial types combined (70.2% correct), a planned comparison was conducted comparing accuracy on the BB trial type to accuracy on the AB, AA, and BA trial types combined. The analysis indicted that accuracy on the BB trial type was worse than on the other three, F(1,30) = 8.87, p = .014, n2 = 0.65.
Because accuracy scores may not be normally distributed, the accuracy scores pooled over the final 5 sessions of correction training were subjected to an arcsine transformation. Overall, accuracy was significantly different from chance, t(10) = 3.25, p = .009, d = 2.06, and a one-way repeated-measures analysis of variance that was performed on accuracy on the four sequences was significant, F(3,10) = 52.91, p < .0001, n2 = 1.24. Similarly, arcsine-transformed accuracy on the BB trial type was compared to transformed accuracy on the AB, AA, and BA, trial types combined and that effect was also significant, F(1,10) = 35.81, p = .0001, n2 = 1.08.
Two other post hoc analyses were performed on the correction data. If the pigeons were making their choices based on the last color seen, we asked if they were sensitive to the non-reinforcement they received for incorrect choice of the BB trial type. To accomplish this, we scored the BB trial types as if they were correct (58.6% correct). We then combined those data with accuracy on the AB trial types (66.5% correct) and compared those scores to accuracy on the AA and BA trial types combined (80.8% correct). If the pigeons were not sensitive to the first stimulus seen, there should have been no difference. Although the pigeons appeared to be somewhat sensitive to the effect of the first stimulus seen, the difference was not statistically significant, F(1,10) = 2.97, p = .12, n2= 0.23. Finally, given the tendency to choose based on the last stimulus seen, and because the AB sequence was unique, we compared accuracy on the AB sequence with the reverse-scored accuracy on the BB sequence. We did this to see if the identity of the first stimulus seen had an effect on the pigeons’ choice, but again the difference was not significant, F < 1. Thus, overall, the pigeons did not appear to be sensitive to the first color seen.
Discussion
Initial responding to the AB sequence discrimination early in noncorrection training appears to have been based on a bias to choose the response alternative associated with the three non-AB sequences. This occurred, in spite of the fact that because there were three times as many AB sequence trials as each of the others, there should not have been a bias to make the non-AB sequence response. The increase in accuracy on AB sequence trials and decrease in accuracy on BB sequence trials is an indication that several of the pigeons were learning to make their choice based on the stimulus last seen.
The present research identifies a problem in assessing an animal’s ability to acquire a simple two-stimulus sequence. In the typical procedure, the target sequence AB is associated with one response, R1, and the other combinations of those stimuli (AA, BB, and BA) are associated with another response, R2. If one controls for the frequency of the two responses, a high level of accuracy can be achieved by responding based on the last stimulus seen. This alternative to learning the sequence may interfere with learning the R1 response to the AB sequence alone.
Ghirlanda et al. (2017) concluded that it may not be possible for animals to learn to make a specific response to one sequence of two stimuli (e.g., AB) and make a different response to the three other sequences (BB, AA, and BA) of those two stimuli. They reported that although humans acquire such a discrimination readily, non-human animals do not. To account for the difficulty that nonhuman animals have in acquiring a sequence discrimination, Ghirlanda et al. (2017) developed a model based on rapid stimulus forgetting. According to this model, the problem that animals have in discriminating the AB sequence from the BB, AA, and BA sequences is they rapidly forget the first stimulus seen. That is, they see it but they cannot keep in memory long enough to use it after seeing the second stimulus. In the absence of memory for the first stimulus, discrimination of the AB sequence from the BB sequence should be difficult. On the other hand, discrimination of the AB sequence from the AA and BA sequences should be much easier. That prediction is quite consistent with the results of the present experiment, but the mechanism proposed by Ghirlanda et al. is somewhat different from what we propose here. According to the Ghirlanda et al. model, the nature of the first stimulus should have some effect on the sequence discrimination. Thus, because the AA sequence does not include a B stimulus, it should be easier to discriminate from the AB sequence than the BA sequence. As one can see from the results presented in Fig. 2, there is no evidence for such a difference.
Although Weisman et al. (1980) did find a small initial difference in learning between the AA and BA sequences in their first experiment, they found no such difference in their second experiment. Of course, in cases in which there is no effect on accuracy of the first stimulus seen, one could argue that memory for the first stimulus had already declined sufficiently that it no longer had an effect on the discrimination. When effects of the first stimulus do occur, the Ghirlanda et al. model can account for them. However, the last-stimulus-seen account we are proposing is more parsimonious and it accounts for most of the results found.
When animals adopt a response tendency that is different from the ideal (or the sequence learning intent of the experimenter), as Ghirlanda et al. (2017) suggest, it may reflect a limit on the animal’s capacity. Alternatively, we suggest that it may reflect an idiosyncrasy of the procedure used to assess the ability to discriminate. That is by choosing the response alternatives based on a simpler characteristic of the stimulus sequence such as the last stimulus seen, animals may achieve a level of accuracy that may be “good enough.” In other words, under some conditions an animal may get “trapped” by a simpler rule that leads to considerably better than chance accuracy. In the present experiment, sorting the stimulus sequences by the color last seen would result in accuracy not much worse than sorting the stimulus sequences by AB versus BB, AA, and BA. That level of reinforcement may be sufficient to interfere with learning of the sequence task, the way it is defined.
Most of the pigeons learned to perform better than chance by adopting a simpler strategy than learning the target sequence. For some pigeons, however, accuracy never deviated substantially from chance. This is surprising because if one ignores the first stimulus, the task becomes spatial match to sample with 83% reinforcement for correct responding. Assuming that pigeons can learn such a task, it would suggest that in the sequence learning task, the first stimulus in the sequence has some general interfering effect on the second stimulus in the sequence.
Interestingly, in spite of this inherent bias in the sequence learning procedure, Weisman et al. (1980), using an operant go/no-go procedure, found that pigeons did, in fact, acquire the sequence-learning discrimination. Certain differences in their procedure from that used here, as well as that used by Lind et al. (2023), should be noted. Weisman et al. included a third event (X) in their design that consisted of the absence of a stimulus, and their training involved all combinations of the three stimuli, with only the AB pair associated with reinforcement. To somewhat compensate for the large number of stimulus sequences not associated with reinforcement (BB, AA, XX, BA, XA, XB, AX, BX), in each 13 trial block, they included one each of the nonreinforced sequences, one nonreinforced presentation of the AB sequence, and four presentations of the reinforced AB sequence. Thus, within a block of trials, there were nine nonreinforced trials and four reinforced trials.
In addition, with the Weisman et al. (1980) procedure, following presentation of each pair of stimuli, pecks to a white key were used to determine the discrimination ratio (pecks following each non-reinforced pair divided by pecks following the AB pair). Although the pigeons responded mostly to the white key that followed the AB sequence, they also responded between 20% and 40% as much to the other sequence in which the B stimulus appeared last. That is, their pigeons too showed a tendency to peck more when the last stimulus was the same as the last stimulus in the target AB pair.
It is not clear why the go/no-go procedure should be better able to show evidence of sequence learning than the two-alternative forced-choice procedure. One could argue that in the case of the go/no-go procedure there are also two correct responses. R1 is pecking following the AB sequence, and R2 is the absence of pecking following the BB, AA, and BA sequences (as well as following the XA, XB, AX, BX, and XX sequences). It may be, however, that the asymmetry between pecking and not pecking made the R1 and R2 responses more discriminable. It is also possible that the differential outcomes (a reinforcer following most of the AB sequences but none following the other sequences) facilitated acquisition (see e.g., Peterson and Trapold 1980). Furthermore, it is not clear how the additional trials involving the X stimulus (no stimulus) affected the pigeons’ ability to isolate the target AB trials. It is clear, however, that with the two-alternative forced-choice procedure, learning to sort the trials in terms of the last stimulus seen can result in better than chance responding, and that strategy may result in a bias that interferes with pigeons learning the sequence task as defined.
If nonhuman animals adopt a nonoptimal rule such as sorting trials according to the last stimulus seen, how is it that, unlike the pigeons (and other animals), humans avoid learning such a biasing rule that is considered correct on most, but not all trials (Lind et al. 2023). The answer may be that humans have had a long history of learning to judge any trial on which they are incorrect as evidence that they should reject the rule they had been using. That is, at least in our culture, we have learned that there is always a rule that should provide 100% reinforcement. Thus, humans’ ability to show criterial accuracy on this task may reflect their prior history of not accepting a strategy that gets them only close to 100% correct. Humans may have learned to attend to correcting the trials on which they have been incorrect. Specifically, if they had begun using the last color as the basis of their choice, they would have made errors on BB trials. By then attending to BB trials, they would learn that what made those trials incorrect was that the sorting rule, based on the last stimulus seen, was not always correct. It was incorrect when the last stimulus, B, was also preceded by stimulus B. Humans may thus strive to correct the few remaining errors made, whereas animals may not discriminate the difference in reinforcement or be as motivated to correct the small number of errors that occur.
Data availability
Programs and data can be obtained from the first author at zentall@uky.edu.
References
Atance CM, O’Neill DK (2001) Episodic future thinking. Trends Cogn Sci 5:533–539
Christiansen MH, Chater N (2016) The now-or-never bottleneck: a fundamental constraint on language. Behav Brain Sci 39(e62). https://doi.org/10.1017/S0140525X1500031X
Ghirlanda S, Lind J, Enquist M (2017) Memory for stimulus sequences: a divide between humans and other animals? Royal Soc Open Sci 4(6):161011. https://doi.org/10.1098/rsos.161011PMID: 28680660; PMCID: PMC5493902
Hambrick DZ, Burgoyne AP, Altman EM (2020) Problem solving and intelligence. In: Sternberg RJ (ed) Cambridge Handbook of Intelligence, 2nd edn. Cambridge University Press, New York, 553–579
James W (1899) Talks to teachers on psychology–and to students on some of life’s ideals. Metropolitan Books/Henry Holt and Company. https://doi.org/10.1037/10814-000
Lind J, Vinken V, Jonsson M, Ghirlanda S, Enquist M (2023) A test of memory for stimulus sequences in great apes. PLoS ONE 18(9):e0290546. https://doi.org/10.1371/journal.pone.0290546
Peterson GB, Trapold MA (1980) Effects of altering outcome expectancies on pigeons’ delayed conditional discrimination performance. Learn Motiv 11:267–288
Roberts WA (1972) Short-term memory in the pigeon: effects of repetition and spacing. J Exp Psychol 94:74–83. https://doi.org/10.1037/h0032796
Skinner BF (1950) Are theories of learning necessary? Psychol Rev 57(4):193–216. https://doi.org/10.1037/h0054367
Tulving E (2002) Episodic memory: from mind to brain. Annual Review of Psychology, 53, 1–25. https://doi.org/10.1146/annurev.psych.53.100901.135114. PMID: 11752477
Ullman MT (2004) Contributions of memory circuits to language: the declarative/procedural model. Cognition 92:231–270. https://doi.org/10.1016/j.cognition.2003.10.008
Weisman R, Wasserman E, Dodd P, Larew MB (1980) Representation and retention of two-event sequences in pigeons. J Exp Psychol Anim Behav Process 6(4):312–325
Zentall TR (1973) Memory in the pigeon: retroactive inhibition in a delayed matching task. Bull Psychon Soc 1:126–128 Competing interests: The authors have no competing interests to declare
Funding
None.
Author information
Authors and Affiliations
Contributions
D.P. wrote the computer program, supervised the experiment, ran the analyses, and edited the ms. T.Z. designed the experiment, made up the figures, and wrote and edited the ms.
Corresponding author
Ethics declarations
Ethical approval
The pigeons were maintained in accordance with protocol #2020–3675 approved by the Institutional Animal Care and Use Committee at the University of Kentucky.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zentall, T.R., Peng, D.N. The problem with two-event sequence learning by pigeons. Anim Cogn 27, 63 (2024). https://doi.org/10.1007/s10071-024-01906-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10071-024-01906-1