Abstract
Living organisms routinely navigate their surroundings in search of better conditions, more food, or to avoid predators. Typically, animals do so by integrating sensory cues from the environment with their locomotor apparatuses. For single cells or small organisms that possess motility, fundamental physical constraints imposed by their small size have led to alternative navigation strategies that are specific to the microscopic world. Intriguingly, underlying these myriad exploratory behaviours or sensory functions is the onset of periodic activity at multiple scales, such as the undulations of cilia and flagella, the vibrations of hair cells, or the oscillatory shape modes of migrating neutrophils. Here, I explore oscillatory dynamics in basal microeukaryotes and hypothesize that these active oscillations play a critical role in enhancing the fidelity of adaptive sensorimotor integration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ability to move in a seemingly purposeful, goal-oriented way even in the presence of dynamic environmental perturbations is sometimes assumed to be a sign of cognition or even intelligence. Indeed, the most ancestral purpose of the nervous system of living organisms may have been to control self-motion (i.e., motility) (Wan and Jékely 2021; Jékely et al. 2015; De Wiljes et al. 2015; Keijzer et al. 2013). Pioneering studies in diverse animal species have demonstrated how multiple sensory cues, vision, olfaction, audition, magnetoreception, etc. are integrated actively by the organism’s sensorimotor system to regulate behaviour and improve navigational performance (Vickers 2000; Collett and Graham 2004; Geva-Sagiv et al. 2015). Typically, distinct modalities of sensory input are encoded in different regions of the brain (and by different types of neurons) that together provide the animal with essential information about its location within the environment, the layout, or ‘map’ of its surroundings—including any potential obstacles, its speed of movement, trajectory heading, and the precise orientation of its own ‘self’ in three-dimensional space (Collett and Graham 2004; Frost and Mouritsen 2006; Toledo et al. 2020).
Compare and contrast this to the microscopic realm, with no paucity of examples of goal-oriented navigation strategies found in diverse microbial species. That microorganisms, including single cells, also have the capacity to receive, to process, and to perceive information, potentially also integrating multiple distinct sensory cues, is becoming increasingly evident (Lyon 2015). These traits were openly recognised by influential early researchers (Jennings 1902; Binet 1894), but it is only with the advent of recent bioimaging technologies (high-speed cameras, microscopes with high resolving power, etc.), that these processes and hypotheses can be queried and critiqued in detail.
The act of navigation (of an organism or animal) should be distinguished from simple migration from point A to point B, for the former includes active sampling of the environment, and successful negotiation of unpredictable or unfamiliar terrains. In both air and water, behaving animals routinely exploit and adapt to complex chemical and fluid dynamic landscapes in response to a (possibly patchy or transient) nutrient or odour source. Sampling is generally not a random process but in some cases has been shown to obey a closed-loop motor programme. Oscillations of hair-like appendages such as whiskers and antennae function to replenish the local air or water to increase olfactory function (Claverie et al. 2022). Oscillations of motile cilia in the zebrafish nose have been shown to produce flows that improve the temporal resolution of olfactory sensing (Reiten et al. 2017). Steering or directional reorientations can emerge as a consequence of the feedback between oscillations that alter the amount of exposure to the environmental stimulus, and the stimulus that can in turn perturb the dynamics of the oscillator. Such a mechanism has been demonstrated in the Drosophila larval crawling behaviour, which exhibits lateral oscillations involving significant left-to-right sweeping movements of its head (containing the chemosensory organ of the larva) (Wystrach et al. 2016; Gomez-Marin et al. 2011). In two phylogenetically distant species of navigating ants, it was shown that they exhibit movement patterns with a persistent, underlying oscillatory component that is at least tenfold slower than that of the ants’ natural stride frequency (Clement et al. 2023). In mammals, the so-called theta oscillations that appear in electrical recordings of brain activity have been shown to play a key role in spatial navigation and memory, perhaps to promote the synchronization of neuronal firing (Bender et al. 2015).
Beyond examples relevant for navigation and sensing, it is clear that inherently oscillatory phenomena are highly widespread in biological systems as a signature of a complex dynamical system (Kruse and Jülicher 2005). Spontaneous waves and oscillations may simply reflect the underlying dynamic organisation or boundary conditions of the system, and do not have a purpose. For instance, spontaneous noisy oscillations have been observed in minimal reconstituted acto-myosin networks under elastic loading, comprising a single actin filament and only tens of myosin motors (Plaçais et al. 2009). In other cases, oscillations do have a clear functional role. In the clock-and-wavefront model, molecular segmentation clocks—oscillating patterns of gene expression—in the pre-somitic mesoderm underlie vertebrate somitogenesis, leading to cellular differentiation (e.g., into endothelium, dermis, skeletal muscle, tendon, and cartilage) (Oates et al. 2012; Chou et al. 2022). In the syncytial protist Physarum polycephalum, famed for its ability to solve mazes and respond to chemical cues, cellular migration is tightly coupled to the vascular network of the organism, resulting in pronounced contraction–relaxation cycles and oscillatory patterns of fluid flow. These flows are conjectured to provide an alternative route to memory and information storage that is distinct from the evolution of nervous systems (Boussard et al. 2021; Alim et al. 2017; Reid 2023).
The purpose of this essay is to explore and clarify the putative functional role of active oscillations in the context of microscale navigation strategies, particularly in aquatic environments. I will discuss the necessity and inevitability of oscillatory behaviours as a means to generate locomotion in an inertia-less regime (relevant for very small organisms), and the physical consequences of these actions, particularly for sensing. I hope that this motivates new insights into how organisms, both macroscopic and microscopic, make important decisions about where to go and how to get there, and how these capabilities originated in the first instance in the microscopic world.
Oscillatory dynamics at the microscopic scale
Oscillations as a means of moving
From the flapping tails of fish, to the rhythmic writhing of eels and snakes, diverse animal species achieve locomotion by stereotyped and periodic actuation of body parts. When the wavelength of the gait is shorter than the body length, the swimmer is generally known as an oscillatory swimmer, if longer, it may instead be classified as an undulatory swimmer (Smits 2019). Cyclic behaviours are natural or indeed inevitable, since they are needed to return the body part or appendage to its initial position, so that the next step, wave, or gait cycle can begin anew (Purcell 1977; Gallistel et al. 1983) (Fig. 1).
At the microscopic scale, organisms rely mainly on viscous drag, rather than inertia, for self-propulsion. For macroscopic animals, this is the difference between swimming in honey, rather than in water. Here, geometry dictates motion, and the gait is kinematic. It is not the rate of shape change, or how fast an appendage deforms, but the extent of deformation that is most important (Hatton et al. 2013). Simply put, the swimmer’s speed is linearly proportional to its shape velocity (Shapere and Wilczek 1989). For example, consider a three-link swimmer, first hypothesized by Purcell (1977), whose time-dependent shape is completely characterised by a vector of joint angles \({\varvec{\alpha }}=(\alpha _1,\alpha _2)\). The swimmer’s shape velocity would then be given by \(\textrm{d}{\varvec{\alpha }}/\textrm{d}t\). A \(3\times 2\) matrix, called the connection, then relates the swimmer’s speed in \((x,y,\theta )\)-space with \({\varvec{\alpha }}\) (Fig. 1b). The swimming velocity is maximised when the shape velocity and the connection vectors are parallel. In a properly parameterised shape space, such as \((\alpha _1,\alpha _2)\), a gait (i.e., sequence of cyclic shape changes), produces closed curves that enclose a finite area, leading to propulsion. Net swimming is a consequence of many small (infinitesimal) translations and rotations generated by the organism’s shape changes, summed over time. For the Purcell swimmer, an undulatory gait sequence where the two angles are 1/4-cycle phase-lagged will trace out a circle in shape-space (Fig. 1c), to produce net displacement in the positive-x direction (Hatton et al. 2013; Wiezel and Or 2016). Such a planar 3-link swimmer has been shown to be controllable in 2D in a control theoretic sense, meaning that every final state is reachable from some initial state, and that the optimal strategy for swimming is indeed to to execute periodic sequences of strokes (Giraldi et al. 2013). More generally, symmetry is broken—and a transition to a more ordered but asymmetrical state is achieved—in the deformation sequence, either by propagating a wave, which is inherently directional, or by generating an asymmetric beat pattern (Fig. 1d). This is a consequence of the lack of time-dependence in the Stokes’ equations, which govern motion in a viscosity-dominated regime. Playing the same stroke pattern backwards in time solves the same equations and leads to the same motion, but in reverse. This is known as Stokes’ reversibility, which microswimmers must overcome to achieve net propulsion.
Conceptual summary of geometric mechanics in action at the microscale. a Limit cycle dynamics (red arrow) underlie diverse motility mechanisms; these are often stable to perturbations (black arrows). b Oscillations are natural due to the need to create a periodic gait. The displacement maps linearly to the shape velocity via a local connection matrix (here plotted for forward motion), where shape is represented by a 2D shape-space of angles \(\alpha =(\alpha _1,\alpha _2\)). The distance travelled is then proportional to the contour integral along the gait in shape-space, and hence the frequency of gait actuation. [Figure adapted from Hatton et al. (2013), Gong et al. (2016), and courtesy of Daniel Goldman.] A basic artificial three-link swimmer generates a non-reciprocal sequence of shapes that can achieve net swimming (c) [red vertical reference line shows net rightward displacement), while the biflagellate alga Chlamydomonas actuates its two cilia in a coordinated breaststroke (d) (data obtained by the author) (color figure online)
This basic principle is exploited by various microorganisms (as well as bioinspired artificial or robotic swimmers) that can actuate different appendage types (flagella, archaella, or cilia) (Bondoc-Naumovitz et al. 2023; Diaz et al. 2021; Lim et al. 2022). Whereas bacterial flagella and archaella (the flagella of archaeal species) are rigid helices that can only be rotated from one end, cilia are much larger, structurally more complex, and only found in eukaryotes where they display more degrees of mechanical freedom (Wan and Jékely 2021; Beeby et al. 2020). Although these appendage types differ fundamentally in their mode of operation and actuation mechanism, they have in common a slender shape that experiences anisotropic drag as segmental elements are moved through a fluid. In diverse organisms, appendages are arranged in various configurations, and actuated in a periodic manner through fluids and other complex friction-dominated environments (Fig. 2a, b).
Even motile cell types that do not have permanent appendages employ temporary actin-based protrusions called pseudopodia which grow and retract around the cell, to produce the symmetry breaking and body deformations that are necessary for net motility. As fish keratocytes crawl on substrates, the coupling between elasticity and adhesion gives rise to distinctive oscillations in shape akin to a ‘bipedal’ gait (Barnhart et al. 2010). Other eukaryotic cells that routinely navigate through tubes and confined spaces exhibit increased directional persistence in tight confinement than when unconstrained. In an endothelial cell line, physical confinement increased the levels of intracellular Ca\(^{2+}\), which regulates an underlying molecular clock (with negative feedback) that produces oscillations in RhoA GTPase activity and alters cell migration speed (Lee et al. 2022). Coherent cell-scale actin flows can help polarise animal cells for directional movement (Reversat et al. 2020), but may not be the dominant mechanism for cells migrating in physiological 3D matrices. In such matrix networks, migrating cells exhibited cyclic deformations and speed oscillations that can be captured by a model of the cell as a pair of oscillating force dipoles (one on either side of the nucleus) that breaks symmetry via a phase shift in time (Godeau et al. 2022) (Fig. 2c). In these types of dynamics, the net migration speed over one cycle is proportional to the gait frequency.
More unusual migration strategies are found in diverse protists. The process by which euglenid species produce extreme but periodic deformations of their entire bodies is known as metaboly, where flexible strips of pellicle, a flexible proteinaceous layer covering the outer cell membrane, are sheared helically past each other (Arroyo et al. 2012). This produces a relatively slow (only 1–2 \(\upmu\)m/s) swimming speed, compared to what is typical of cilia-driven motility (often on the order of 100 \(\upmu\)m/s). In Plasmodium, single-celled parasites of the phylum Apicomplexa, sporozoites exhibit gliding motility on substrates, producing striking circular trajectories on glass slides, but helical paths when moving in 3D. The physical mechanism for this is unclear but is thought to be related to the basic chiral shape of the cell (Yahata et al. 2021). The periodicity of the trajectories appears to be strongly correlated with the oscillations in intracellular Ca\(^{2+}\) levels (Carey et al. 2014). The model biflagellate alga Chlamydomonas also glides on surfaces. This form of motility repurposes an ancient mechanism known as Intraflagellar Transport (IFT) by which proteins are transported back and forth along the axoneme by molecular motors (kinesins and dyneins) (Bloodgood 2010). This mechanism does not rely on the propagation of bends along the axoneme, but instead membrane glycoproteins carried by the IFT machinery transiently adhere to the substrate. The motors responsible for cargo transport then exert pulling forces on the paused glycoproteins, resulting in gliding along the direction of the flagellum (Shih et al. 2013). Whole cells glide with both flagella fully outstretched (Fig. 2c), but how can symmetry be broken? It turns out that Ca\(^{2+}\) signalling is compartmentalised and coordinated between the two flagella. In the trailing flagellum, elevated Ca\(^{2+}\) promotes the disengagement of IFT particles from the flagella to enable the leading flagellum to win the tug of war and facilitate smooth gliding. On adherent surfaces, repetitive Ca\(^{2+}\) elevations prevent the accumulation of paused IFT particles, thereby indirectly regulates the adhesion force (Bloodgood 2010; Fort et al. 2021).
Oscillations as a means of sensing
In tracing the deep evolutionary origins of microorganisms, the ancestral cilium undoubtedly played an important role in sensing (Quarmby and Leroux 2010; Jékely 2009), though it is unclear which aspect of cilium function arose first: motility or sensing. Though motile cilia have become specialised for propelling flows, they are nevertheless adorned with ion channels and diverse capabilities to transduce and sense environmental signals. In the absence of external cues, the orderly beat of some motile cilia can be highly robust to biochemical noise due to background fluctuations, maintaining a sharply peaked frequency distribution in such species. In Chlamydomonas, changes in the cilia beat frequency spectrum are a direct readout of the cell’s physiological state, with slow oscillations in the instantaneous beat frequency occurring on a much slower timescale than flagellar beating itself, possibly due to fluctuations in intracellular Ca\(^{2+}\) (Wan and Goldstein 2014). When the cilium is perturbed mechanically, its preferred or baseline beat dynamics can be recovered quickly, within characteristic time, and in a load-dependent manner (Wan and Goldstein 2014; Klindt et al. 2016).
Some motile cilia display repetitive movements that are distinct from normal ciliary beating in that they lack large-amplitude bending, instead undergoing periodic, small-amplitude vibrations. This vibrational mode has been reported in green algae (Wan and Goldstein 2018) and in the hyperoscillations of sperm flagella (Brokaw 2009; Kamimura and Kamiya 1989). Conversely, while most sensory (sometimes known as primary) cilia have lost their ability to beat, some specialised cilia types found in the vertebrate node (Yuan et al. 2015) and in the mechanosensory organs of marine invertebrates also vibrate at defined frequencies (Bezares-Calderón et al. 2020). Cilia vibrations are associated with the acute mechanosensory capabilities of marine algae (Wan and Goldstein 2018) and in the unusual auditory system of tree crickets (Mhatre 2015). Similarly, faint sounds are known to be actively amplified by mechanosensory hair bundles in the mammalian inner ear, by exploiting an oscillatory instability (where the physical system suddenly exhibits oscillations as a certain critical threshold in forcing is reached, see also further discussions in the section “Oscillations at system criticality”) (Camalet et al. 2000). The existence of an underlying frequency signature seems to be a universal feature in the above examples, and suggests that oscillations could contribute to sensory function.
One possibility lies in the ability of certain cells to generate their own signals, especially in cases where the external signal is weak (e.g., shallow chemical gradients) or cell size is small (Wan and Jékely 2021; Dusenbery 1998). Since oscillatory inputs (in contrast to steady-state signals) have high-information content, cells can overcome sensory limitations by generating self-oscillations and then convolving the self-originated signals with environmental information. According to an emerging perspective, crawling eukaryotic cells (e.g., immune cells) exploit self-generated gradients for steering and chemotaxis (Insall et al. 2022; Tweedy et al. 2020). Indeed, the same cell can both produce and respond to the signalling molecule or chemical, such as during autocrine signalling. When this occurs with a temporal feedback or relay (Insall et al. 2022), complex dynamics can emerge including coordinated behaviour across a population of cells.
Regardless of whether or not the motile cells have temporary protrusions or permanent appendages, they often follow saltatory trajectories reminiscent of the lateral oscillations of the behaving ants I described above. These dynamics may be further coupled to various types of taxes (Ehrengruber et al. 1996). In its single-cell amoeba stage, Dictyostelium undergoes repetitive shape changes as it crawls, which coincides with force-dependent changes in adhesion, to produce a stepping action. Even in the absence of external cues, this stepping largely conforms to a left–right–left alternating pattern of pseudopod extension with a period of 1–2 min (Bosgraaf and Van Haastert 2009). This non-random pattern of always turning away from the last turn results in a diffusive zigzag search pattern with long directional persistence, which may optimise sampling efficiency in unseen environments (Yang et al. 2011; Li et al. 2008). In biophysical models, zigzag trajectories where a particle hops with equal probability left or right but with some fixed or preferred turning angle produce large spatial diffusion constants and enhanced area of coverage (Schimansky-Geier et al. 2005). This search strategy has also been hypothesized to confer an evolutionary advantage in some organisms, such as in the case of the optimal foraging behaviour of the plankton Daphnia (Garcia et al. 2007).
Taken together, we may hypothesize that single cells (indeed sub-cellular structures) harness self-initiated oscillatory activity to create sensory reafference by generating significant relative motion between the self and the environment, and thereby achieve heightened sensing. These active sensing modalities may parallel well-established processes in the macroscopic world, as found in many marine invertebrates (Jékely et al. 2021) and in weakly electric fish (Hofmann et al. 2013). I will return to this point in the next section in the specific case of freely swimming microorganisms with helical motility patterns (Fig. 2d).
Oscillations at system criticality
From a physical perspective, living systems are often said to be poised at criticality (Mora and Bialek 2011; Kinouchi and Copelli 2006), treading that metaphorical fine line between order and disorder. This precarious yet useful state of existence ensures that such systems are simultaneously robust to noise, and yet also able to respond to environmental changes in a timely manner. Excessive order can make a dynamical system too predictable or too slow to respond, yet the other extreme of too little organisation can pose challenges to the normal functions of a cell.
In microscale systems, ensembles of active biomolecular components (motors, filaments, muscles, sarcomeres, etc.) have to negotiate complex viscoelastic environments and time-dependent stresses. When system parameters evolve dynamically in time, this can often lead to bifurcations from steady-state dynamics to oscillatory behaviour (Tamayo et al. 2022; Kruse and Jülicher 2005; Westendorf et al. 2013). This can happen even in a minimal system comprising a single motor applying a follower force (i.e., applied tangentially) at the tip of an elastic filament that is pinned at one end. As the applied force increases, a Hopf bifurcation results, leading to an oscillatory instability (De Canio et al. 2017). This kind of ‘flutter’ instability was first described in macroscopic, high-Reynolds-number systems (think garden hose, or flapping flags) (Xie et al. 2016; Gregory and Paidoussis 1966; Argentina and Mahadevan 2005). The microscale version has since been proposed recently as a possible molecular mechanism for the onset of oscillations in beating cilia and flagella (Ling et al. 2018; Woodhams et al. 2022). The control of ciliary beating remains a very active topic of study and a major open question in the field, which we do not discuss at length here. Several other mechanisms have been studied extensively in the literature, with varying levels of experimental support (Lindemann and Lesich 2021; Brokaw 2009; Sartori et al. 2016).
While oscillatory dynamics can emerge naturally in this way, living systems likely evolved to harness control of this phenomenon for certain functions. A prominent demonstration of this is the sensing of sound in the vertebrate hearing organ, by bundles of mechanically activated stereocilia in the inner ear. In-depth analyses have suggested that self-oscillations amplify hearing responses close to the bifurcation threshold for instability (Martin et al. 2001). This is an active, nonlinear process that requires a constant flow of energy into the system to sustain the oscillations. Frequency tuning of self-oscillations further modifies the properties of the hair bundles, rendering them selectively sensitive to certain signal-frequency ranges. The exact mechanism and site of sensory amplification in hair cells is debated (Mhatre 2015), and it remains to be elucidated whether oscillations are an artefact or mechanism of active sensing in different systems, including the insect chordotonal organ (Göpfert and Hennig 2016).
The existence of oscillations also permits the possibility of their entrainment by other oscillators, cycles, or signalling events, notably in neural dynamics. The firing of an individual neuron is itself poised at criticality, as rationalised in seminal work by Hodgkin and Huxley (1952). The dynamics can be captured by a system of differential equations involving the time rate of change of the membrane potential. The system is excitable; intuitively, this means that even small (but above threshold) fluctuations in the balance of ionic fluxes can result in large deviations of the membrane potential or spiking event, where membrane depolarisation is followed by repolarisation. For certain parameter combinations, firing can occur at regular intervals, and the single neuron can thus be considered as an excitable oscillator (Stiefel and Ermentrout 2016; Rinzel and Ermentrout 1998). In larger animals, networks, circuits, or systems of neurons underpin diverse and complex sensorimotor rhythms, such as walking, running, or hunting. The rich dynamics of neural synchronization between different brain regions, or even avalanche events, are critical to information propagation and the proper organisation of brain function. The activity of oscillators can therefore enable entrainment on a global or collective scale, and in certain cases, as we have seen in the previous section, amplify sensory functions.
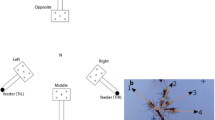
Oscillations in microscale navigation are ubiquitous, but do they serve a purpose? a, b Motility trajectories of two different strains of Chlamydomonas reinhardtii, a model biflagellate alga, observed under the microscope. c Examples of motility strategies that rely on the active oscillations of body parts: swimming using cilia and flagella, gliding on substrates, bipedal crawling of keratocytes, and eukaryotic cell migrating as a pair of oscillating force dipoles. (Red arrows indicate direction of migration.) d Examples of sensing strategies that rely on the active oscillations of cilia: stochastic aerotaxis of choanoflagellates (the colony makes as many correct as incorrect turns), deterministic (helical) klinotaxis in many small eukaryotes, and sperm cells (the superhelical paths of the organism tend to align with the stimulus direction) (color figure online)
Navigation strategies of microswimmers
Next, we discuss the significance of active oscillations, which characterise the self-propulsion strategies of most microscopic organisms, in the context of two distinct navigation algorithms, one stochastic and the other deterministic.
-
a.
Stochastic search in a colonial choanoflagellate
Due to their small physical size, most prokaryotes (with some exceptions) are severely constrained by thermal rotational diffusion and are therefore unable to sustain any given migration or swimming path for a sufficiently long time for effective signal integration (Berg 1984; Wan and Jékely 2021). Consequently, such microbes can only access stochastic search strategies, such as the classic run-and-tumble chemotaxis of E. coli. The latter is not generally a true taxis as the direction of swimming does not follow the chemical gradient; instead, the cell suppresses the tendency to tumble (associated with path reorientation) when migrating up the gradient towards a chemoattractant (Berg and Purcell 1977). Over long times, this biases the movement of the organism without the need for steering. Another common strategy is to bias the reorientation angle after tumbling, instead of the run speed, depending on the direction of the gradient (Saragosti et al. 2011). Detailed modelling and inference methods can be used to estimate the rotational diffusion associated with the active motility strategies of such micron-sized cells (Seyrich et al. 2018).
A recent biophysical study revealed that the choanoflagellate Salpingoeca rosetta adopts a stochastic strategy for aerotaxis—the biased migration towards oxygen (Kirkegaard et al. 2016a). This is somewhat surprising, since colonial eukaryotes like S. rosetta are certainly large enough to steer effectively (Wan and Jékely 2021), so it is unclear why in the transition to multicellularity, choanoflagellates have not evolved to steer. The answer may well lie in the stochastic morphology of S. rosetta: a facultative multicellular organism, where the number of cells per colony is highly variable, encompassing unicellular as well as multicellular forms, and the ability to switch between forms. A stochastic search may be a consequence of both the highly stochastic beating of the individual flagella, and the inability of the flagella to coordinate their activity over the entire colony, with no obvious relationship between colony size and fidelity of stochastic navigation (Kirkegaard et al. 2016b). In contrast, green algae of similar size do coordinate their flagella for processes such as phototaxis, as we shall discuss in the next case study. Genomic evidence places choanoflagellates as an important sister group to the metazoa (King 2004; Ros-Rocher and Brunet 2023). It is of interest that these organisms appear to have retained the more ancestral stochastic navigation strategy rather than evolve additional mechanisms for intercellular flagellar coordination or communication.
Another important feature is the demonstration of logarithmic sensing in choanoflagellate aerotaxis. Using a combination of fast gas-exchange experiments and simulations, Kirkegaard et al. (2016a) showed that S. rosetta effectively discriminates oxygen gradients in low-concentration environments by logarithmic sensing. In a coarse-grained, Keller–Segel-type description of the population dynamics, the drift velocity can be written as \(V = v_\text {drift}\tanh \left( \frac{\gamma }{C}\frac{\textrm{d}C}{\textrm{d}y}\right) \textbf{e}_y\), for a perfectly vertical gradient in the \(\textbf{e}_y\)-direction, where \(\gamma\) is a constant and \(\tanh\) is the hyperbolic tangent function. Here, the speed is tuned not to the absolute gradient of the signal \(\nabla C\) but instead to the change in signal relative to the background \(\nabla C/|C|\) (in other words, to \(\nabla \log C)\). (Here, \(\nabla\) denotes the Del operator, so that \(\nabla C = \textrm{d}C/\textrm{d}y\) in 1D, or \(\nabla C = (\partial C/\partial x, \partial C/\partial y, \partial C/\partial z)\) in 3D.) In this interpretation of the experimental data, the individual swimming colony modulates its swimming direction stochastically in response to the gradient, rather than its swimming speed v. At long times, the propulsion strategy leads to \(v_\text {drift}=v\left\langle \sin \theta \right\rangle\), where \(\theta\) denotes the orientation of the individual colony and \(\left\langle \cdot \right\rangle\) denotes the ensemble average. The distribution of turning angles in time \(\Delta t\): \(\Delta \phi = |\phi (t+\Delta t)-\pi /2|-|\phi (t)-\pi /2|\) was measured experimentally and confirmed to be centred at \(\Delta \phi =0\) (Kirkegaard et al. 2016a). This produces a noisy search pattern with as many wrong turns as right turns (Fig. 2d).
This type of nonlinear sensing is found in many sensory systems operating both at the low- and high-signal limit (fold-change detection); this is analogous to why the sensitivity of the human ear to sounds is also quantified on a logarithmic scale (in decibels). From the perspective of saturating sensors at the microscale (Endres and Wingreen 2008), the error in the measured concentration gradient scales with the absolute concentration itself, so the limit of detection must decrease with absolute concentration. This is also known as Weber’s law of sensory perception (Stevens 1957). The physiological need to operate at the ‘sensory limit’ can significantly constrain the design of the signalling system that underlies these sensory functions, particularly at cellular scale, where thermal fluctuations are significant. Although the biochemical transduction pathway responsible for choanoflagellate aerotaxis has not yet been elucidated at a molecular level, it is possible that it shares some features with E. coli chemotaxis, where robustness is suggested to emerge from network design, and rather insensitive to exact system parameters (Barkai and Leibler 1997).
-
b.
Deterministic steering in algae and sperm
In the above, the individual S. rosetta colony’s noisy helical trajectory (on the timescale of stochastic flagellar oscillations) was not accounted for, but yet could still play a role in the eventual stochastic search strategy. For over 100 years, helical navigation has been conjectured to be important for navigation in motile microeukaryotes (Jennings 1902), where the interplay between the organism’s intrinsic helical swimming path and the resulting oscillatory sampling of a directional signal leads to deterministic steering towards the stimulus, this time a true taxis (Crenshaw 1993). Recent experimental and theoretical studies have demonstrated deterministic steering in sperm chemotaxis (Kashikar et al. 2012; Jikeli et al. 2015; Kaupp and Alvarez 2016) as well as in the phototaxis strategies of diverse green algae (Cortese and Wan 2021; Drescher et al. 2010). Steering is possible despite active fluctuations occurring at the scale of individual flagella, which can surpass thermal fluctuations (Ma et al. 2014; Wan and Goldstein 2014).
Photosynthetic green algae, such as Chlamydomonas reinhardtii and Volvox carteri, have optimised their sensorimotor system for steering towards light (Yoshimura and Kamiya 2001). Unlike S. rosetta, both species coordinate their flagella (either in a pair or in a collective) to produce smooth helical swimming. Using a variety of experimental approaches to deliver pulsed light stimuli, the strongest frequency response in the motor system of these organisms was shown to be elicited at the self-rotation frequency (of helical swimming) (Leptos et al. 2023; Drescher et al. 2010; Schaller et al. 1997; Hegemann 1997). Moreover, in the biflagellate C. reinhardtii, the response delay time from signal perception to modulation of flagella activity also coincides with the timescale required for the cell’s mean flagellar beat plane, which lags behind the eyespot location, to rotate in line with the stimulus direction (Fig. 2). Another alga, Euglena gracilis, exhibits an adaptive phototaxis strategy and follows strikingly polygonal trajectories (varying in order from 3 to 5) in response to sudden increase in light intensity (Tsang et al. 2018). In all cases, the relationship between the sensor and detector can be encoded by \(I(t) = I_0(\textbf{I}\cdot {\hat{\textbf{s}}})H(\textbf{I} \cdot \hat{\textbf{s}})\), where \(\textbf{I}\) is the stimulus direction in the lab-frame, \((\textbf{I}\cdot {\hat{\textbf{s}}})\) is the stimulus projected onto the direction of maximal sensitivity of the detector (e.g., eyespot direction), and H is a Heaviside step function, which is equal to 0 when the argument is negative and 1 otherwise. The step function accounts for periodic shading of the detector as the detected intensity is zero until the eyespot has rotated to face the stimulus. When the organism oscillates along helical paths, the perceived stimulus also oscillates. Depending on the nature of the motor apparatus, I(t) then modifies the swimming behaviour accordingly.
To fulfil other forms of taxes, a dedicated directional sensor may not be required, as in the case of chemotaxis of animal sperms. Although spatial heterogeneities in the distribution of chemoreceptors and ion channels cannot be ruled out. In the extensively studied system of the sea urchin Arbacia punctulata, freely swimming sperm were placed in chemical landscapes and their 3D behavioural responses to caged resact, a potent chemoattractant for sea urchin sperm, released by photolysis were tracked by digital holography (Jikeli et al. 2015). In a simplified model of these measurements, the basic 3D flagellar waveform can be characterised by a constant twist and curvature \(\kappa (s,t) = K_0+A\cos (\omega t-\lambda s)\) at time t and arclength s, where A is the wave amplitude, \(\omega /\lambda\) the wave speed, and \(K_0\) the mean curvature. Such a beat produces realistic superhelical paths (as well as twisted ribbons). Due to the periodic trajectory, a spatial concentration profile of chemoattractant \(C(\textbf{x},t)\) is converted to a temporal stimulus at the location \(\textbf{x}(t)\) of the sperm via \(S(t) = C(\textbf{x}(t))\); this intracellular signal then induces a change in the path curvature \(\kappa (t)\), with an adaptive time-delay (Friedrich and Jülicher 2007; Kashikar et al. 2012). For step-on responses (swimming up the chemical gradient), it has been shown both theoretically and experimentally that a small periodic modulation of the flagellum curvature leads to the smooth alignment of the helix axis \(\textbf{h}\) with the gradient in a deterministic rather than stochastic manner. That is, the change in direction of the helix axis \(d\textbf{h}/dt\) is biased towards the direction of \(\nabla _\perp C = \nabla C-(\nabla C\cdot \textbf{h})\textbf{h}\), the component of the chemical gradient that is perpendicular to the initial helix orientation. The turning response is also Ca\(^{2+}\)-dependent, but not solely on the intracellular calcium concentration (Wood et al. 2007). Finally, we note that periodic dynamics are also involved in 2D exploration, for example with sperm swimming near boundaries or interfaces, often along circular trajectories instead of helices.
Despite the intrinsic differences between exploration of chemical versus photonic cues (binding of disperse molecules versus interception of photons), both sperm chemotaxis and algal phototaxis are examples of helical klinotaxis (Friedrich and Jülicher 2007; Crenshaw 1993). In both cases, the axis of the swimming helix gradually reorients until it is aligned with that of the stimulus gradient (Fig. 2d): while the baseline gradient controls the global response magnitude, the fast oscillations occur at the frequency of flagellar beating. By combining sensory adaptation (high-pass) with temporal integration (low-pass), klinotactic steering operates as a bandpass filter (Yoshimura and Kamiya 2001) This can result in reafferent sensorimotor coupling, as introduced in the previous section, where a feedback between the self-generated oscillatory signal and the stimulus produces a deterministic steering response with in-built error correction akin to a servomechanism.
The adaptive coupling between a microswimmer’s angular rotation and the vectorial stimulus is important for navigation. This simple mechanism is exemplified by its action in single microswimmers, but also exists in the neural circuits of animals (Jékely et al. 2008; Fraenkel and Gunn 1941; McHenry and Strother 2003; Kane et al. 2013). The self-generated gradients produced by an organism’s self-movement may also form the basis of other, lesser-explored navigation behaviours, such as rheotaxis and gravitaxis of planktonic marine larvae (Wheeler et al. 2019; Takeda-Sakazume et al. 2022; Jékely et al. 2021). Intriguingly, directional navigation can even emerge spontaneously in the case of oscillatory rheotaxis (upswimming against an external flow) in active artificial microswimmers (Dey et al. 2022), where oscillations are controlled by the interaction between the boundaries and the self-generated gradients in surfactant produced by the otherwise spherical, isotropic droplets. For a navigating animal, its undulatory movements can produce lateral oscillations that enable temporal sampling, and the ability to distinguish between step-up and step-down responses to stimuli. In movement ecology, achieving a fine balance between exploration (side-to-side movement) versus exploitation (direct migration from A to B) could be integral to the stochastic search patterns of migrating animals and even humans (Clement et al. 2023; Bartumeus and Levin 2008; Volchenkov et al. 2013), often with signatures of multiple oscillation frequencies in the dynamics.
Discussion
Coordinating oscillations for motility
We have demonstrated that at the cellular scale relevant for microscale navigation, oscillations and oscillatory dynamics emerge naturally, and are a powerful means to both encode and transduce information in space and time. They enable small cells and organisms to embody a mechanical intelligence about their environment whilst soliciting minimal involvement from the ‘brain’ or central-processor equivalent. By embedding periodic signatures in patterns of locomotion and sensing, they allow for coordinated or synchronized actions between different parts of the same organism, or between different organisms. While the ubiquity of oscillatory processes is undeniable, care should be taken not to ascribe function in cases where there may be none. Careful theoretical analyses and experimental studies are needed to determine whether true function exists in any given context.
In the course of evolution, active oscillatory dynamics in cells may have arisen accidentally but subsequently became preferentially selected. For example, motile cilia beating may have emerged when the first molecular motors that were originally intended for surface or intraflagellar transport (Bloodgood 2010) spontaneously coordinated within an elastic axoneme to produce self-sustained oscillations (Jülicher and Prost 1997). Over time, cells then repurposed these cilia oscillations and diverse stroke patterns exclusively for motility generation, due to their vastly superior function when navigating through fluids and other complex environments. Here, swimming relies on the cyclic deformation of parts of the organism’s body (e.g., the appendages), as indeed do the locomotion strategies of most terrestrial and aquatic animal species (Dickinson et al. 2000). The ubiquity of helical swimming in microbial navigation can be explained by noting that for periodic deformations in the body frame of the swimmer in a low-Reynolds number environment, superhelical swimming trajectories are the most general solution that satisfies the correct equations of fluid dynamics, since any asymmetric stroke will result in a non-zero translation and rotation after each stroke cycle (Rossi et al. 2017). This naturally leads to two distinct timescales encoded in the organism’s movement patterns, a fast timescale on the order of the self-deformations (e.g., cilia beat frequency), and a usually slower timescale over which the ’slow’ helical turns are completed (Fig. 2). For Chlamydomonas, typical rotation frequencies are 1–2 Hz, while flagellar beat frequencies are 50–70 Hz. As we have highlighted in the previous section, helical swimming has further downstream benefits for stimulus-oriented navigation. Helical klinotaxis obeys a similar fundamental physical principle, whether for organisms with largely symmetric body shape (Cortese and Wan 2021; Leptos et al. 2023; Jékely et al. 2008), or those with obvious body asymmetries (Rossi et al. 2017; Jikeli et al. 2015). In the case of helical chemotaxis, successful gradient following occurs for a wide range of system parameters (Lange and Friedrich 2021), again demonstrating underlying system robustness. Thus, this mechanism of converting spatial to (self-generated) temporal gradients is highly robust to noise, and forms the basis of a versatile and evolutionarily ancestral search strategy that is agnostic to the precise morphology of the organism.
Wherever multiple appendages are involved, how these are coordinated and controlled in different organisms remains an intriguing open question (Wan 2018; Gilpin et al. 2020). Not only are the control strategies system-dependent, they are also dynamic and sensitive to environmental changes (Quaranta et al. 2015; Klindt and Friedrich 2016). In many-cilia systems, hydrodynamic interactions can dominate the interactions between neighbouring appendages (Brumley et al. 2014; Vilfan and Jülicher 2006; Elgeti and Gompper 2013), whereas in systems with a small number of precisely positioned cilia, active intracellular mechanisms are important (Guo et al. 2021; Wan and Goldstein 2016). Continuing to draw parallels between microbial and animal navigation, we can identify deep similarities between microbial motility, in which modular cellular components are coupled dynamically with capacity for error correction, and motor programming in animal movement (Grillner et al. 1991; Collins and Richmond 1994). The limbs or segments of a locomoting animal’s body often maintain deterministic phase relationships; surprisingly, this is also observed in the gaits of algal flagellates (Wan and Goldstein 2016), and to a lesser extent in the stochastic gaits of walking ciliates that use cirri (bundled cilia) to move on surfaces (Larson et al. 2022) This raises the possibility that the ancestral analogue of central pattern generators (CPGs)—ubiquitous in animal locomotion Marder and Bucher (2001)—may have arisen in the first instance in the single-celled eukaryote, purely from the necessity of coordinating multiple oscillators for motility and navigation (Wan 2020). These basic mechanisms are then elaborated in the complex nervous system architectures of animals, where intrinsically oscillatory appendage actuation obeys different network topologies, and regulation by servomotors (Cheng 2022, 2023). CPG-inspired circuits also underlie the many designs for robots modelled on the locomotor patterns of animals Ijspeert (2008).
Motility, cognition, and perception of the self
We conclude our discourse by returning to the very notion that motility may have predated cognition itself (Fig. 3). As we seek to understand and trace the deep evolutionary origins of animal cognition, there is growing consensus that neural systems first evolved to coordinate self-movement, while sensory modulation arose only later (Goodrich 2010). Central to biological cognition is an organism’s perception of time, which may be embodied in the multiplexed oscillations of the body, effectively turning the body into a clock. At the macroscale, this has been demonstrated in rodents that were given a reward if approached after a fixed interval: instead of remaining at rest between events, the animals developed stereotyped motor routines that improved the accuracy of time-keeping (Safaie et al. 2020). The notion that ‘each oscillatory cycle is a temporal processing window’ (Buzsáki 2006) does not have to apply exclusively to neural systems; in contrast to static or constant-amplitude signals, a wave or a fluctuating signal can be intrinsically directional. I suggest that these features enable even microscopic organisms to perceive the arrow of time.
By exploiting the intrinsic properties of active systems close to a critical point, living systems can access both stable dynamics yet also rapid responses to external cues, as we saw in the oscillatory instabilities of beating cilia. Further, oscillatory inputs may have high information content, with the ability to buffer as well as filter signals. These conceptual traits are paralleled in excitable oscillators and neurons (Stiefel and Ermentrout 2016), as they are in the dynamics of the motile single cell. For decades, the ciliate Paramecium has been referred to as ‘a swimming neuron’ (Kung and Saimi 1985), with renewed interest in exploring the extent of this interpretation in recent years (Brette 2021; Elices et al. 2023). By generating and controlling repetitive spikes, or routines in activity, single cells can keep time, generate, and store memories, and even learn (Fig. 3). This type of learning typically manifests at timescales (short) relevant for cellular physiology, in contrast to inherited traits or generational adaptation which occurs over evolutionary timescales (long). These hidden intracellular signatures often manifest as changes in the observed motile behaviours of these organisms. Examples include associative learning in the avoidance behaviour of Stentor (Rajan et al. 2023; Gershman et al. 2021) and galvanotaxis in three species of free-living amoebae (Carrasco-Pujante et al. 2021), the stochastic hunting strategy of the protist Lacrymaria, in which space is repetitively and densely sampled (Coyle et al. 2019), and the rhythmic dynamics of ciliate escape from dead ends (Kunita et al. 2014), though the biochemical origins of the latter is disputed (Ishikawa and Kikuchi 2018).
Excitable membrane dynamics and other threshold phenomena may have contributed significantly to the origin of eukaryotes, which co-evolved with diversification of cilia function and increased complexity in the cognitive capabilities of cells. Thus, with motility, so came cognition; we should be left in no doubt that the microscopic world holds unique and invaluable insights into how sensory and cognitive processes are encoded in living systems.
Data availability
Not applicable.
References
Alim K, Andrew N, Pringle A, Brenner MP (2017) Mechanism of signal propagation in Physarum polycephalum. Proc Natl Acad Sci 114(20):5136–5141
Argentina M, Mahadevan L (2005) Fluid-flow-induced flutter of a flag. Proc Natl Acad Sci 102(6):1829–1834
Arroyo M, Heltai L, Millán D, DeSimone A (2012) Reverse engineering the euglenoid movement. Proc Natl Acad Sci 109(44):17874–17879
Barkai N, Leibler S (1997) Robustness in simple biochemical networks. Nature 387(6636):913–917
Barnhart EL, Allen GM, Jülicher F, Theriot JA (2010) Bipedal locomotion in crawling cells. Biophys J 98(6):933–942
Bartumeus F, Levin SA (2008) Fractal reorientation clocks: linking animal behavior to statistical patterns of search. Proc Natl Acad Sci 105(49):19072–19077
Beeby M, Ferreira JL, Tripp P, Albers S-V, Mitchell DR (2020) Propulsive nanomachines: the convergent evolution of archaella, flagella and cilia. FEMS Microbiol Rev 44(3):253–304
Bender F, Gorbati M, Cadavieco MC, Denisova N, Gao X, Holman C, Korotkova T, Ponomarenko A (2015) Theta oscillations regulate the speed of locomotion via a hippocampus to lateral septum pathway. Nat Commun 6(1):8521
Berg HC (1984) Random walks in biology. Princeton University Press, Princeton
Berg H, Purcell E (1977) Physics of chemoreception. Biophys J 20(2):193–219
Bezares-Calderón LA, Berger J, Jékely G (2020) Diversity of cilia-based mechanosensory systems and their functions in marine animal behaviour. Philos Trans R Soc B Biol Sci 375(1792):20190376
Binet A (1894) The psychic life of micro-organisms : a study in experimental psychology. The Open Court Publishing Company, Chicago
Bloodgood RA (2010) Sensory reception is an attribute of both primary cilia and motile cilia. J Cell Sci 123(4):505–509
Bondoc-Naumovitz KG, Laeverenz-Schlogelhofer H, Poon RN, Boggon AK, Bentley SA, Cortese D, Wan KY (2023) Methods and measures for investigating microscale motility. Integr Comp Biol (Jun 19):icad075
Bosgraaf L, Van Haastert PJM (2009) The ordered extension of pseudopodia by amoeboid cells in the absence of external cues. PLoS One 4(4):e5253
Boussard A, Fessel A, Oettmeier C, Briard L, Döbereiner H-G, Dussutour A (2021) Adaptive behaviour and learning in slime moulds: the role of oscillations. Philos Trans R Soc B Biol Sci 376(1820):20190757
Brette R (2021) Integrative neuroscience of Paramecium, a “Swimming Neuron”. eneuro 8(3):ENEURO.0018-21.2021
Brokaw CJ (2009) Thinking about flagellar oscillation. Cell Motil Cytoskelet 66(8):425–436
Brumley DR, Wan KY, Polin M, Goldstein RE (2014) Flagellar synchronization through direct hydrodynamic interactions. eLife 3:e02750
Buzsáki G (2006) 111A system of rhythms: from simple to complex dynamics. In: Buzsáki G (ed) Rhythms of the brain. Oxford University Press
Camalet S, Duke T, Jülicher F, Prost J (2000) Auditory sensitivity provided by self-tuned critical oscillations of hair cells. Proc Natl Acad Sci 97(7):3183–3188
Carey AF, Singer M, Bargieri D, Thiberge S, Frischknecht F, Ménard R, Amino R (2014) Calcium dynamics of P. lasmodium berghei sporozoite motility: sporozoite calcium dynamics. Cell Microbiol 16(5):768–783
Carrasco-Pujante J, Bringas C, Malaina I, Fedetz M, Martínez L, Pérez-Yarza G, Dolores Boyano M, Berdieva M, Goodkov A, López JI, Knafo S, De la Fuente IM (2021) Associative conditioning is a robust systemic behavior in unicellular organisms: an interspecies comparison. Front Microbiol 12:707086
Cheng K (2022) Oscillators and servomechanisms in orientation and navigation, and sometimes in cognition. Proc R Soc B Biol Sci 289(1974):20220237
Cheng K (2023) From representations to servomechanisms to oscillators: my journey in the study of cognition. Anim Cogn 26(1):73–85
Chou K-T, Lee D-YD, Chiou J-G, Galera-Laporta L, Ly S, Garcia-Ojalvo J, Süel GM (2022) A segmentation clock patterns cellular differentiation in a bacterial biofilm. Cell 185(1):145-157.e13
Claverie N, Steinmann T, Bandjee MJ, Buvat P, Casas J (2022) Oscillations for active sensing in olfaction: bioinspiration from insect antennal movements. Bioinspir Biomimet 17(5):055004
Clement L, Schwarz S, Wystrach A (2023) An intrinsic oscillator underlies visual navigation in ants. Curr Biol 33(3):411-422.e5
Collett TS, Graham P (2004) Animal navigation: path integration, visual landmarks and cognitive maps. Curr Biol 14(12):R475–R477
Collins JJ, Richmond SA (1994) Hard-wired central pattern generators for quadrupedal locomotion. Biol Cybern 71(5):375–385
Cortese D, Wan KY (2021) Control of helical navigation by three-dimensional flagellar beating. Phys Rev Lett 126(8):088003
Coyle SM, Flaum EM, Li H, Krishnamurthy D, Prakash M (2019) Coupled active systems encode an emergent hunting behavior in the unicellular predator Lacrymaria olor. Curr Biol CB 29(22):3838-3850.e3
Crenshaw HC (1993) Orientation by helical motion-III. Microorganisms can orient to stimuli by changing the direction of their rotational velocity. Bull Math Biol 55(1):231–255
De Canio G, Lauga E, Goldstein RE (2017) Spontaneous oscillations of elastic filaments induced by molecular motors. J R Soc Interface 14(136):20170491
De Wiljes OO, Van Elburg RAJ, Biehl M, Keijzer FA (2015) Modeling spontaneous activity across an excitable epithelium: support for a coordination scenario of early neural evolution. Front Comput Neurosci 9:110
Dey R, Buness CM, Hokmabad BV, Jin C, Maass CC (2022) Oscillatory rheotaxis of artificial swimmers in microchannels. Nat Commun 13(1):2952
Diaz K, Robinson TL, Aydin YO, Aydin E, Goldman DI, Wan KY (2021) A minimal robophysical model of quadriflagellate self-propulsion. Bioinspir Biomimet 16(6):066001
Dickinson MH, Farley CT, Full RJ, Koehl MAR, Kram R, Lehman S (2000) How animals move: an integrative view. Science 288(5463):100–106
Drescher K, Goldstein RE, Tuval I (2010) Fidelity of adaptive phototaxis. Proc Natl Acad Sci 107(25):11171–11176
Dusenbery DB (1998) Spatial sensing of stimulus gradients can be superior to temporal sensing for free-swimming bacteria. Biophys J 74(5):2272–2277
Ehrengruber MU, Deranleau DA, Coates TD (1996) Shape oscillations of human neutrophil leukocytes: characterization and relationship to cell motility. J Exp Biol 199(4):741–747
Elgeti J, Gompper G (2013) Emergence of metachronal waves in cilia arrays. Proc Natl Acad Sci 110(12):4470–4475
Elices I, Kulkarni A, Escoubet N, Pontani L-L, Prevost AM, Brette R (2023) An electrophysiological and kinematic model of Paramecium, the “swimming neuron’’. PLoS Comput Biol 19(2):e1010899
Endres RG, Wingreen NS (2008) Accuracy of direct gradient sensing by single cells. Proc Natl Acad Sci USA 105(41):15749–15754
Fort C, Collingridge P, Brownlee C, Wheeler G (2021) Ca2+ elevations disrupt interactions between intraflagellar transport and the flagella membrane in Chlamydomonas. J Cell Sci 134(3):jcs253492
Fraenkel GS, Gunn DL (1941) The orientation of animals, kinesis, taxes and compass reactions. Ann Entomol Soc Am 34(3):690–690
Friedrich BM, Jülicher F (2007) Chemotaxis of sperm cells. Proc Natl Acad Sci 104(33):13256–13261
Frost BJ, Mouritsen H (2006) The neural mechanisms of long distance animal navigation. Sens Syst 16(4):481–488
Gallistel C (1983) The organization of action: a new synthesis. Psychology Press
Garcia R, Moss F, Nihongi A, Strickler JR, Göller S, Erdmann U, Schimansky-Geier L, Sokolov IM (2007) Optimal foraging by zooplankton within patches: The case of Daphnia. In: BIOCOMP2005 Special Issue, 207(2):165–188
Gershman SJ, Balbi PE, Gallistel CR, Gunawardena J (2021) Reconsidering the evidence for learning in single cells. eLife 10:e61907
Geva-Sagiv M, Las L, Yovel Y, Ulanovsky N (2015) Spatial cognition in bats and rats: from sensory acquisition to multiscale maps and navigation. Nat Rev Neurosci 16(2):94–108
Gilpin W, Bull MS, Prakash M (2020) The multiscale physics of cilia and flagella. Nat Rev Phys 2(2):74–88
Giraldi L, Martinon P, and Zoppello M (2013) Controllability and optimal strokes for N-link microswimmer. In: 52nd IEEE Conference on Decision and Control, pages 3870–3875. Journal Abbreviation: 52nd IEEE Conference on Decision and Control
Godeau AL, Leoni M, Comelles J, Guyomar T, Lieb M, Delanoë-Ayari H, Ott A, Harlepp S, Sens P, Riveline D (2022) 3D single cell migration driven by temporal correlation between oscillating force dipoles. eLife 11:e71032
Gomez-Marin A, Stephens GJ, Louis M (2011) Active sampling and decision making in Drosophila chemotaxis. Nat Commun 2(1):441
Gong CI, Goldman D, Choset H (2016) Simplifying gait design via shape basis optimization. In: Robotics: science and systems XII. Robotics: Science and Systems Foundation 2016
Goodrich BG (2010) We do, therefore we think: time, motility, and consciousness. Rev Neurosci 21(5):331–361
Göpfert MC, Hennig RM (2016) Hearing in Insects. Annu Rev Entomol 61(1):257–276
Gregory RW, Paidoussis MP (1966) Unstable oscillation of tubular cantilevers conveying fluid I. Theory. Proc R Soc Lond Ser A Math Phys Sci 293(1435):512–527
Grillner S, Wallen P, Brodin L, Lansner A (1991) Neuronal network generating locomotor behavior in lamprey: circuitry, transmitters, membrane properties, and simulation. Annu Rev Neurosci 14:149–199
Guo H, Man Y, Wan KY, Kanso E (2021) Intracellular coupling modulates biflagellar synchrony. J R Soc Interface 18(174):20200660
Hatton RL, Ding Y, Choset H, Goldman DI (2013) Geometric visualization of self-propulsion in a complex medium. Phys Rev Lett 110(7):078101
Hegemann P (1997) Vision in microalgae. Planta 203(3):265–274
Hodgkin AL, Huxley AF (1952) A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol 117(4):500–544
Hofmann V, Sanguinetti-Scheck JI, Künzel S, Geurten B, Gómez-Sena L, Engelmann J (2013) Sensory flow shaped by active sensing: sensorimotor strategies in electric fish. J Exp Biol 216(13):2487–2500
Ijspeert AJ (2008) Central pattern generators for locomotion control in animals and robots: a review. Neural Netw 21(4):642–653
Insall RH, Paschke P, Tweedy L (2022) Steering yourself by the bootstraps: how cells create their own gradients for chemotaxis. Trends Cell Biol 32(7):585–596
Ishikawa T, Kikuchi K (2018) Biomechanics of Tetrahymena escaping from a dead end. Proc R Soc B Biol Sci 285(1873):20172368
Jékely G (2009) Evolution of phototaxis. Philos Trans R Soc B Biol Sci 364(1531):2795–2808
Jékely G, Colombelli J, Hausen H, Guy K, Stelzer E, Nédélec F, Arendt D (2008) Mechanism of phototaxis in marine zooplankton. Nature 456(7220):395–399
Jékely G, Keijzer F, Godfrey-Smith P (2015) An option space for early neural evolution. Philos Trans R Soc B Biol Sci 370(1684):20150181
Jékely G, Godfrey-Smith P, Keijzer F (2021) Reafference and the origin of the self in early nervous system evolution. Philos Trans R Soc B Biol Sci 376(1821):20190764
Jennings HS (1902) Studies on reactions to stimuli in unicellular organisms. ix.-on the behavior of fixed infusoria (Stentor and Vorticella), with special reference to the modifiability of protozoan reactions. Am J Physiol-Leg Content 8(1):23–60. https://doi.org/10.1152/ajplegacy.1902.8.1.23
Jikeli JF, Alvarez L, Friedrich BM, Wilson LG, Pascal R, Colin R, Pichlo M, Rennhack A, Brenker C, Kaupp UB (2015) Sperm navigation along helical paths in 3D chemoattractant landscapes. Nat Commun 6(1):7985
Jülicher F, Prost J (1997) Spontaneous oscillations of collective molecular motors. Phys Rev Lett 78(23):4510–4513
Kamimura S, Kamiya R (1989) High-frequency nanometre-scale vibration in ‘quiescent’ flagellar axonemes. Nature 340(6233):476–478
Kane EA, Gershow M, Afonso B, Larderet I, Klein M, Carter AR, de Bivort BL, Sprecher SG, Samuel ADT (2013) Sensorimotor structure of Drosophila larva phototaxis. Proc Natl Acad Sci 110(40):E3868–E3877
Kashikar ND, Alvarez L, Seifert R, Gregor I, Jäckle O, Beyermann M, Krause E, Kaupp UB (2012) Temporal sampling, resetting, and adaptation orchestrate gradient sensing in sperm. J Cell Biol 198(6):1075–1091
Kaupp UB, Alvarez L (2016) Sperm as microswimmers-navigation and sensing at the physical limit. Eur Phys J Spec Top 225(11–12):2119–2139
Keijzer F, van Duijn M, Lyon P (2013) What nervous systems do: early evolution, input-output, and the skin brain thesis. Adapt Behav 21(2):67–85
King N (2004) The unicellular ancestry of animal development. Dev Cell 7(3):313–325
Kinouchi O, Copelli M (2006) Optimal dynamical range of excitable networks at criticality. Nat Phys 2(5):348–351
Kirkegaard JB, Bouillant A, Marron AO, Leptos KC, Goldstein RE (2016a) Aerotaxis in the closest relatives of animals. eLife 5:e18109
Kirkegaard JB, Marron AO, Goldstein RE (2016b) Motility of colonial choanoflagellates and the statistics of aggregate random walkers. Phys Rev Lett 116(3):038102
Klindt GS, Ruloff C, Wagner C, Friedrich BM (2016) Load response of the flagellar beat. Phys Rev Lett 117(25):258101
Kruse K, Jülicher F (2005) Oscillations in cell biology. Curr Opin Cell Biol 17(1):20–26
Kung C, Saimi Y (1985) Chapter 3 Ca2+ channels of paramecium: a multidisciplinary study. Curr Top Membr Transp 23:45–66
Kunita I, Kuroda S, Ohki K, Nakagaki T (2014) Attempts to retreat from a dead-ended long capillary by backward swimming in Paramecium. Front Microbiol 5:270
Lange S, Friedrich BM (2021) Sperm chemotaxis in marine species is optimal at physiological flow rates according theory of filament surfing. PLoS Comput Biol 17(4):e1008826
Larson BT, Garbus J, Pollack JB, Marshall WF (2022) A unicellular walker controlled by a microtubule-based finite-state machine. Curr Biol 32(17):3745-3757.e7
Lee SH, Hou JC, Hamidzadeh A, Yousafzai MS, Ajeti V, Chang H, Odde DJ, Murrell M, Levchenko A (2022) A molecular clock controls periodically driven cell migration in confined spaces. Cell Syst 13(7):514-529.e10
Leptos KC, Chioccioli M, Furlan S, Pesci AI, Goldstein RE (2023) Phototaxis of Chlamydomonas arises from a tuned adaptive photoresponse shared with multicellular Volvocine green algae. Phys Rev E 107(1):014404
Li L, Nørrelykke SF, Cox EC (2008) Persistent cell motion in the absence of external signals: a search strategy for eukaryotic cells. PLoS One 3(5):e2093
Lim S, Du Y, Lee Y, Kumar Panda S, Tong D, Khalid Jawed M (2022) Fabrication, control, and modeling of robots inspired by flagella and cilia. Biosinpir Biomim 18(1):011003
Lindemann CB, Lesich KA (2021) The many modes of flagellar and ciliary beating: Insights from a physical analysis. Cytoskeleton 78(2):36–51
Ling F, Guo H, Kanso E (2018) Instability-driven oscillations of elastic microfilaments. J R Soc Interface 15(149):20180594
Lyon P (2015) The cognitive cell: bacterial behavior reconsidered. Front Microbiol 6:264
Ma R, Klindt GS, Riedel-Kruse IH, Jülicher F, Friedrich BM (2014) Active phase and amplitude fluctuations of flagellar beating. Phys Rev Lett 113(4):048101
Marder E, Bucher D (2001) Central pattern generators and the control of rhythmic movements. Curr Biol 11(23):R986–R996
Martin P, Hudspeth AJ, Jülicher F (2001) Comparison of a hair bundle’s spontaneous oscillations with its response to mechanical stimulation reveals the underlying active process. Proc Natl Acad Sci 98(25):14380–14385
McHenry M, Strother J (2003) The kinematics of phototaxis in larvae of the ascidian Aplidium constellatum. Mar Biol 142(1):173–184
Mhatre N (2015) Active amplification in insect ears: mechanics, models and molecules. J Comp Physiol A 201(1):19–37
Mora T, Bialek W (2011) Are biological systems poised at criticality? J Stat Phys 144(2):268–302
Oates AC, Morelli LG, Ares S (2012) Patterning embryos with oscillations: structure, function and dynamics of the vertebrate segmentation clock. Development 139(4):625–639
Plaçais P-Y, Balland M, Guérin T, Joanny J-F, Martin P (2009) Spontaneous oscillations of a minimal actomyosin system under elastic loading. Phys Rev Lett 103(15):158102
Purcell EM (1977) Life at low Reynolds-number. Am J Phys 45(1):3–11
Quaranta G, Aubin-Tam M-E, Tam D (2015) Hydrodynamics versus intracellular coupling in the synchronization of eukaryotic flagella. Phys Rev Lett 115(23):238101
Quarmby LM, Leroux MR (2010) Sensorium: the original raison D’etre of the Motile Cilium? J Mol Cell Biol 2(2):65–67
Rajan D, Makushok T, Kalish A, Acuna L, Bonville A, Correa Almanza K, Garibay B, Tang E, Voss M, Lin A, Barlow K, Harrigan P, Slabodnick MM, Marshall WF (2023) Single-cell analysis of habituation in Stentor coeruleus. Curr Biol 33(2):241-251.e4
Reid CR (2023) Thoughts from the forest floor: a review of cognition in the slime mould Physarum polycephalum. Anim Cognit
Reiten I, Uslu FE, Fore S, Pelgrims R, Ringers C, Diaz Verdugo C, Hoffman M, Lal P, Kawakami K, Pekkan K, Yaksi E, Jurisch-Yaksi N (2017) Motile-Cilia-mediated flow improves sensitivity and temporal resolution of olfactory computations. Curr Biol 27(2):166–174
Reversat A, Gaertner F, Merrin J, Stopp J, Tasciyan S, Aguilera J, de Vries I, Hauschild R, Hons M, Piel M, Callan-Jones A, Voituriez R, Sixt M (2020) Cellular locomotion using environmental topography. Nature 582(7813):582–585
Rinzel J, Ermentrout GB (1998) Analysis of neural excitability and oscillations. In: Koch C, Negev I (eds) Methods in neuronal modeling: from synapses to networks. MIT Press. https://nyuscholars.nyu.edu/en/publications/analysis-of-neural-excitability-and-oscillations-2
Ros-Rocher N, Brunet T (2023) What is it like to be a choanoflagellate? Sensation, processing and behavior in the closest unicellular relatives of animals. Anim Cognit
Rossi M, Cicconofri G, Beran A, Noselli G, DeSimone A (2017) Kinematics of flagellar swimming in Euglena gracilis: helical trajectories and flagellar shapes. Proc Natl Acad Sci 114(50):13085–13090
Safaie M, Jurado-Parras M-T, Sarno S, Louis J, Karoutchi C, Petit LF, Pasquet MO, Eloy C, Robbe D (2020) Turning the body into a clock: accurate timing is facilitated by simple stereotyped interactions with the environment. Proc Natl Acad Sci 117(23):13084–13093
Saragosti J, Calvez V, Bournaveas N, Perthame B, Buguin A, Silberzan P (2011) Directional persistence of chemotactic bacteria in a traveling concentration wave. Proc Natl Acad Sci 108(39):16235–16240
Sartori P, Geyer VF, Scholich A, Jülicher F, Howard J (2016) Dynamic curvature regulation accounts for the symmetric and asymmetric beats of Chlamydomonas flagella. eLife 5:e13258
Schaller K, David R, Uhl R (1997) How Chlamydomonas keeps track of the light once it has reached the right phototactic orientation. Biophys J 73(3):1562–1572
Schimansky-Geier L, Erdmann U, Komin N (2005) Advantages of hopping on a zig-zag course. New Horizons Stoch Complex 351(1):51–59
Seyrich M, Alirezaeizanjani Z, Beta C, Stark H (2018) Statistical parameter inference of bacterial swimming strategies. New J Phys 20(10):103033
Shapere A, Wilczek F (1989) Geometry of self-propulsion at low Reynolds number. J Fluid Mech 198(1):557
Shih SM, Engel BD, Kocabas F, Bilyard T, Gennerich A, Marshall WF, Yildiz A (2013) Intraflagellar transport drives flagellar surface motility. eLife 2:e00744
Smits AJ (2019) Undulatory and oscillatory swimming. J Fluid Mech 874:P1
Stevens SS (1957) On the psychophysical law. Psychol Rev 64(3):153–181
Stiefel KM, Ermentrout GB (2016) Neurons as oscillators. J Neurophysiol 116(6):2950–2960
Takeda-Sakazume A, Honjo J, Sasano S, Matsushima K, Baba SA, Mogami Y, Hatta M (2022) Gravitactic Swimming of the planula larva of the coral acropora: characterization of straightforward vertical swimming. Zool Sci 40(1):44–52
Tamayo J, Mishra A, Gopinath A (2022) Ambient fluid rheology modulates oscillatory instabilities in filament-motor systems. Front Phys 10:895536
Toledo S, Shohami D, Schiffner I, Lourie E, Orchan Y, Bartan Y, Nathan R (2020) Cognitive map-based navigation in wild bats revealed by a new high-throughput tracking system. Science 369(6500):188–193
Tsang ACH, Lam AT, Riedel-Kruse IH (2018) Polygonal motion and adaptable phototaxis via flagellar beat switching in the microswimmer Euglena gracilis. Nat Phys 14(12):1216–1222
Tweedy L, Thomason PA, Paschke PI, Martin K, Machesky LM, Zagnoni M, Insall RH (2020) Seeing around corners: cells solve mazes and respond at a distance using attractant breakdown. Science 369(6507):eaay9792
Vickers N (2000) Mechanisms of animal navigation in odor plumes. Biol Bull 198(2):203–212
Vilfan A, Jülicher F (2006) Hydrodynamic flow patterns and synchronization of beating cilia. Phys Rev Lett 96(5):058102
Volchenkov D, Helbach J, Tscherepanow M, Kühnel S (2013) Exploration-exploitation trade-off features a saltatory search behaviour. J R Soc Interface 10(85):20130352
Wan KY (2018) Coordination of eukaryotic cilia and flagella. Essays Biochem 62(6):829–838
Wan KY (2020) Synchrony and symmetry-breaking in active flagellar coordination. Philos Trans R Soc B-Biol Sci 375(1792):20190393
Wan KY, Goldstein RE (2014) Rhythmicity, recurrence, and recovery of flagellar beating. Phys Rev Lett 113(23):238103
Wan KY, Goldstein RE (2016) Coordinated beating of algal flagella is mediated by basal coupling. Proc Natl Acad Sci USA 113(20):E2784–E2793
Wan KY, Goldstein RE (2018) Time irreversibility and criticality in the motility of a flagellate microorganism. Phys Rev Lett 121(5):058103
Wan KY, Jékely G (2021) Origins of eukaryotic excitability. Philos Trans R Soc B-Biol Sci 376:20190758
Westendorf C, Negrete J, Bae AJ, Sandmann R, Bodenschatz E, Beta C (2013) Actin cytoskeleton of chemotactic amoebae operates close to the onset of oscillations. Proc Natl Acad Sci 110(10):3853–3858
Wheeler JD, Secchi E, Rusconi R, Stocker R (2019) Not just going with the flow: the effects of fluid flow on bacteria and plankton. Annu Rev Cell Dev Biol 35:213–237
Wiezel O, Or Y (2016) Using optimal control to obtain maximum displacement gait for Purcell’s three-link swimmer. In: 2016 IEEE 55th Conference on Decision and Control (CDC), pages 4463–4468. Journal Abbreviation: 2016 IEEE 55th Conference on Decision and Control (CDC)
Wood CD, Nishigaki T, Tatsu Y, Yumoto N, Baba SA, Whitaker M, Darszon A (2007) Altering the speract-induced ion permeability changes that generate flagellar Ca2+ spikes regulates their kinetics and sea urchin sperm motility. Dev Biol 306(2):525–537
Woodhams LG, Shen Y, Bayly PV (2022) Generation of ciliary beating by steady dynein activity: the effects of inter-filament coupling in multi-filament models. J R Soc Interface 19(192):20220264
Wystrach A, Lagogiannis K, Webb B (2016) Continuous lateral oscillations as a core mechanism for taxis in Drosophila larvae. eLife 5:e15504
Xie F, Zheng X, Triantafyllou MS, Constantinides Y, Karniadakis GE (2016) The flow dynamics of the garden-hose instability. J Fluid Mech 800:595–612
Yahata K, Hart MN, Davies H, Asada M, Wassmer SC, Templeton TJ, Treeck M, Moon RW, Kaneko O (2021) Gliding motility of Plasmodium merozoites. Proc Natl Acad Sci 118(48):e2114442118
Yang TD, Park J-S, Choi Y, Choi W, Ko T-W, Lee KJ (2011) Zigzag turning preference of freely crawling cells. PLoS One 6(6):e20255
Yoshimura K, Kamiya R (2001) The sensitivity of Chlamydomonas photoreceptor is optimized for the frequency of cell body rotation. Plant Cell Physiol 42(6):665–672
Yuan S, Zhao L, Brueckner M, Sun Z (2015) Intraciliary calcium oscillations initiate vertebrate left-right asymmetry. Curr Biol 25(5):556–567
Acknowledgements
KYW would like to thank the editors of this special issue, Pamela Lyon and Ken Cheng, for the opportunity to present this review.
Funding
KYW is supported by funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme under Grant 853560 (EvoMotion), and from the Biotechnology and Biological Sciences Research Council under Grant BB/W00853X/1.
Author information
Authors and Affiliations
Contributions
KYW wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The author has no competing interests to declare.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wan, K.Y. Active oscillations in microscale navigation. Anim Cogn 26, 1837–1850 (2023). https://doi.org/10.1007/s10071-023-01819-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-023-01819-5