Abstract
Incorporating novel food sources into their diet is crucial for animals in changing environments. Although the utilization of novel food sources can be learned individually, learning socially from experienced conspecifics may facilitate this task and enable a transmission of foraging-related innovations across a population. In anthropogenically modified habitats, bats (Mammalia: Chiroptera) frequently adapt their feeding strategy to novel food sources, and corresponding social learning processes have been experimentally demonstrated in frugivorous and animalivorous species. However, comparable experiments are lacking for nectarivorous flower-visiting bats, even though their utilization of novel food sources in anthropogenically altered habitats is often observed and even discussed as the reason why bats are able to live in some areas. In the present study, we investigated whether adult flower-visiting bats may benefit from social information when learning about a novel food source. We conducted a demonstrator–observer dyad with wild Pallas’ long-tongued bats (Glossophaga soricina; Phyllostomidae: Glossophaginae) and hypothesized that naïve individuals would learn to exploit a novel food source faster when accompanied by an experienced demonstrator bat. Our results support this hypothesis and demonstrate flower-visiting bats to be capable of using social information to expand their dietary repertoire.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Incorporating novel food sources into the dietary repertoire is crucial for animals in order to acquire resources in changing environments. This can be easily observed in anthropogenically altered habitats, where animals expand their niche by exploiting food sources related to humans, including crops, livestock or ornamental plants (Giménez-Anaya et al. 2008; Amit et al. 2013; Kruszynski et al. 2016), by feeding on invasive species (Kottsieper et al. 2019) or even by using intentionally provided feeding stations (Tryjanowski et al. 2015).
While the use of novel food sources can be learned individually, for example by trial-and-error learning, social learning by “observation of, or interaction with, another individual or its products” can facilitate this task (Hoppitt and Laland 2013, p. 4). The mere presence of a conspecific may reduce neophobia or promote explorative behavior and thus facilitate interactions with a novel food source (social facilitation, Zajonc 1965), or learning individuals may benefit from information transfer by participating in the knowledge of a more experienced conspecific (social transmission, Hoppitt and Laland 2013). Regardless of the various mediating mechanisms (Galef and Giraldeau 2001; Galef and Laland 2005), social transmission may spread foraging-related innovations across a population and form local traditions (van Schaik 2010; Laland et al. 2011; Aplin et al. 2013, 2015).
In anthropogenically modified habitats, bats (Mammalia: Chiroptera) can be frequently observed to expand their dietary repertoire with novel food sources. For instance, insectivorous bats may shift their feeding habits in response to introduced prey (Levin et al. 2006), sanguivorous bats may parasitize on pets (Rosa et al. 2013) or livestock (Bobrowiec et al. 2015), while frugivorous and nectarivorous bats may feed on introduced crops (Parry-Jones and Augee 1991; Alpízar et al. 2020) and ornamental plants (Kruszynski et al. 2016; da Silva et al. 2017; Pellón et al. 2021), or may even exploit artificial sugar water resources like hummingbird feeders (Buecher and Sidner 2013; Maguina and Muchhala 2017).
Although bats can learn to utilize novel food sources individually, their gregariousness and longevity may facilitate the use of social learning strategies (Wright 2016). In fact, the use of social information has been documented to influence various foraging decisions in bats (reviewed in Wilkinson and Boughman 1999; Wright 2016; Prat and Yovel 2020) and the possibility of a socially facilitated incorporation of novel food sources into a bat’s dietary repertoire has been experimentally demonstrated in frugivorous and animalivorous species (e.g., Ratcliffe and ter Hofstede 2005; Page and Ryan 2006; Wright et al. 2011; Clarin et al. 2014; O’Mara et al. 2014).
However, comparable experiments are lacking for neotropical flower-visiting bats (Phyllostomidae: Glossophaginae), even though their utilization of novel food sources in anthropogenically modified habitats is often observed and even discussed as the reason why bats are able to live in some areas (Buecher and Sidner 2013; Pellón et al. 2021). Flower-visiting bats habitually feed on nectar and pollen from co-evolved bat-pollinated flowers that often share characteristics described as the chiropterophilous syndrome, including a specific unpleasant scent, good accessibility during hovering flight and the production of large amounts of relatively dilute nectar (von Helversen 1995; Tschapka and Dressler 2002). Food sources such as hummingbird feeders or introduced plants often lack these co-evolved chiropterophilous characteristics, raising the question how individual bats initially learn to exploit them.
Pallas’ long-tongued bats (Glossophaga soricina, Glossophaginae) are medium-sized flower-visiting bats with a large geographical distribution from Mexico to Argentina, inhabiting various habitats ranging from montane cloud-forests over lowland rainforests to deciduous dry forests and savannahs (Alvarez et al. 1991; da Rocha et al. 2018). They can be found in primary forests as well as in heavily influenced anthropogenic areas such as banana monocultures and even within cities, where they are regularly observed to drain hummingbird feeders overnight (Kruszynski et al. 2016; Alpízar et al. 2020; Pellón et al. 2021; personal observation). Although there is experimental evidence that G. soricina readily use social information when searching for new locations of an already known food source (Rose et al. 2016), a social transmission of dietary preferences or information about novel food sources has not been demonstrated yet (Rose et al. 2019).
In the present study, we investigated whether social information can facilitate learning about a novel food source in G. soricina. Therefore, we conducted a classical demonstrator-observer dyad in which a naive focal bat had the task to feed from a novel food source in two different test situations, either together with a demonstrator bat that was already familiar with the food source, or, in order to control for social facilitation effects, together with another naive bat. We hypothesized that due to transmission of social information, focal bats would learn to exploit the novel food source faster when together with the knowledgeable demonstrator bat, as compared to focal bats in the control situation.
Materials and methods
Pre-experimental procedure
The study was conducted in the tropical dry forest of the Santa Rosa National Park, Guanacaste, Costa Rica (UTM: 16P 651137 1198498) in two periods from December 2014 to February 2015 and January to February 2016. We used 37 adult G. soricina (27 males, 10 females) that were caught from the wild with mist or hand nets and identified following the field key by Timm and LaVal (1998). We measured forearm length with a caliper (35.7 ± 0.9 mm, mean ± SD, n = 37) and body mass with a spring balance (10.3 ± 0.6 g, n = 37) (Pesola AG). Teeth characteristics for species identification were examined using a jewelry lens.
Prior to the experiment, focal bats were kept in a flight cage (Hexagon Screen House; Eureka) for at least one complete night (2.3 ± 1.2 nights) where they were fed with an artificial nectar solution (NektarPlus® mixed with tap water 1:5, Nekton GmbH, Pforzheim, Germany) offered from a bowl that was placed slightly elevated on the floor. This nectar solution has a distinct odor, that is different from the odor of sulfur-based volatiles of many bat-pollinated flowers. Bats readily accepted this nectar solution and we did not have to release bats due to refusal to feed. With this pre-experimental captivity period, we allowed bats to habituate to being caged and thus avoided bats focusing on escape during the following experiment.
Demonstrator bats were kept inside the experimental cage for 2.3 ± 1.1 nights (Hexagon Screen House; Eureka) and trained to feed from the novel food source that was later used in the experiment. To minimize the number of used bats and reduce time for training periods in between experimental cycles, we trained a total of four demonstrator bats that were each used for up to three experimental cycles.
Novel food source
As a novel food source, we used custom made black cuboidal boxes of 65 × 65 × 35 mm (l × w × h) that comprised a small protruding opening that allowed bats to access an internal sugar water reservoir with their tongues during hovering flight. The boxes differed substantially from bat-pollinated flowers (cf. Tschapka and Dressler 2002) and from potentially familiar artificial food sources like hummingbird feeders, as well as from the bowl of odorous NektarPlus® solution that was provided during the pre-experimental captivity period. Three of these boxes were mounted on an array at a height of 90 cm and at 50 cm distance from each other (Fig. 1A). The array was placed at one wall of the flight cage and thus very noticeably presented to the bats (Fig. 1B). During the experiment, one box was filled with sugar water (17% sucrose), while two boxes remained empty. With this design, we created a challenging foraging situation for focal bats, reflecting food sources that are not rewarding, like immature inflorescences or drained hummingbird feeders.
Experimental cycle
Just before sunset (10.0 ± 9.9 min), bats were caught from their flight cages and placed in small extra cages without food (modified from PS FÅNGST, IKEA). One hour after sunset, we marked the focal bat by gluing a 2 cm reflective stripe on the tip of its back fur using superglue (Fig. 2) and released it to the experimental cage. Here it was allowed to get used to the marking and the experimental cage for 30 min. Subsequently, we mounted the boxes to the array and the experiment was started, either with releasing the demonstrator bat to the experimental cage in the social transmission situation, or with releasing another naïve bat in the control situation. The experiment lasted for 180 min, while bat flights towards the array were recorded by an infrared sensitive Camcorder (DCR-SR, Sony) under infrared illumination (2 HV L-IRC, Sony). By running the experiment at the beginning of the night, we ensured bats were motivated to search for food, but not food-deprived to the extent that they would resign foraging and enter torpor. We conducted 11 replications of each test situation during 22 evenings over two field seasons and used a total of 37 bats (social transmission situation: 11 focal bats, 4 demonstrator bats; control situation: 11 focal bats, 11 naïve bats). To ensure novelty of the food source, each focal and naïve bat was used in only one of the two test situations. Bat sexes were distributed as best as possible equally among the test situations.
Analysis
From the video footage, bats’ behavior was scored until all bats had fed from the novel food source or the experimental time of 180 min had expired, summing up to a total of 48 h of scored video footage. We counted all examining approaches of bats towards boxes (i.e., approaching a box to closer than one body length combined with a change in direction or flight speed), all hovering flights while feeding, and all unsuccessful feeding attempts at empty boxes. After a focal bat had fed from the rewarding box, we scored an additional five minutes to check whether the newly learned food source was revisited.
To analyze the difference between the two test situations (i.e., social transmission situation with demonstrator present vs. control situation with another naïve bat present), we calculated the percentage of focal bats that successfully learned to feed from the novel food source within experimental time (success rate [%]), and the time span until bats first fed from the novel food source (learning time [min]). If a bat remained unsuccessful, we used the maximal experimental time of 180 min as a conservative approximation of their learning time (cf. Wright et al. 2020). Our design with one rewarding and two unrewarding food sources of the same shape allowed us to additionally gain information on whether focal bats rather learned via the location (i.e., food source visited by demonstrator), or by generalizing the demonstrated behavior towards the other, not demonstrated food sources. For this purpose, we analyzed the location of all first feeding attempts of focal bats.
Statistical analysis was performed in R (v. 3.4.3, R Core Team 2017) using the Rcmdr package by Fox and Bouchet-Valat (2017). We compared learning time between both test situations using non-parametric Wilcoxon rank sum test with continuity correction, and success rate by Pearson’s Chi-squared test. Significance level (α = 0.05) was adjusted for multiple testing using sequential Bonferroni correction. The graph for Fig. 3 was created following Weissgerber et al. (2015).
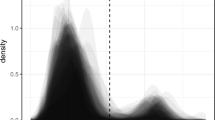
Learning time until bats first fed from the novel food source. In the social transmission situation, focal bats learned significantly faster than in the control situation (Wilcoxon rank sum test with continuity correction, W = 101, p < 0.01 (α = 0.05)). Solid lines depict medians and different symbols of demonstrator bats represent individuals
Results
In the social transmission situation, focal bats learned to feed from the novel food source after 81 ± 59 min (median: 77) with 9 of 11 focal bats being successful (success rate: 82%), while in the control situation, mean learning time was at 165 ± 42 min (median: 180), twice as long with only 2 of 11 focal bats successfully feeding within the experimental time (success rate: 18%) (learning time: Wilcoxon rank sum test with continuity correction, W = 101, p < 0.01 (α = 0.05); success rate: Pearson’s Chi-squared test, χ2 = 8.9091, df = 1, p < 0.01 (α = 0.025)) (Fig. 3). Demonstrator bats visited the food source for the first time 2 ± 3 min after starting the experiment (median: 1) and performed a total of 27.5 ± 18.6 feeding visits (median: 20) before focal bats first fed from the food source or experimental time expired. Each feeding visit lasted for about one second. In both test situations, focal bats remained active throughout experimental time and regularly performed flights inside the cage (Table 1). In the social transmission situation, 60% of first feeding attempts by focal bats were performed at the demonstrated rewarding food source (chance probability: 33%; control situation: 40%). All successful focal bats revisited the novel food source for feeding and, after feeding, five also attempted to feed from a nearby empty box. In the control situation, naive bats behaved similarly to focal bats (success rate: 18%, learning time: 152 ± 59 min (median: 180)).
Discussion
In the presence of a knowledgeable demonstrator, focal bats learned to exploit the novel food source faster and more often than compared to the control situation, which implicates a transmission of information between bats. Since we never tested focal bats alone in our setup, we cannot assess whether the mere presence of a conspecific may have additionally facilitated the learning process, as social facilitation was reported to be beneficial for foraging frugivorous bats (Wright et al. 2020), and also flower-visiting bats are probably bolder and more readily interacting with novel objects if conspecifics are present (Hörmann et al. 2021). Although our experiment was not designed to reveal the actual mechanism behind the observed social transmission, it is likely that the demonstrators’ behavior of approaching and feeding guided the focal bats’ attention towards the array and the novel food source, or that focal bats used the demonstrators’ hovering flights as a cue for the presence of food in this location (cf. local enhancement, Hoppitt and Laland 2013). Our additionally gained data on the location of first feeding attempts may point in this direction. Although the number of first feeding attempts in the control situation is too small for a conclusive comparison, the high probability of focal bats in the social transmission situation to perform their first feeding attempts on the demonstrated rewarding food source may indicate that bats learned rather via the demonstrated location, than by directly generalizing the demonstrator’s behavior to the unrewarding food sources of same shape. Such location-dependent social learning strategies, for instance via following behavior (e.g., Wilkinson 1992) or by eavesdropping on conspecific feeding buzzes (e.g., Barclay 1982) are readily applied by bats searching for new locations of already known food sources. However, flower-visiting bats should also be well suited to apply location-dependent strategies when socially learning about actual food characteristics, since nectar sources such as flowers are usually not removed by a demonstrators’ visit. After the initial learning about a particular novel food source at one location, there is no doubt that flower-visiting bats are able to generalize this knowledge and recognize the same type of food source also at different locations (von Helversen 2004; Thiele and Winter 2005). This is in line with our observation that within 5 min after successfully feeding, five focal bats also attempted to feed on one of the nearby unrewarding boxes. In contrast, for predatory or frugivorous species learning about novel food sources via location is probably not as suitable, since prey or fruits are usually removed by the act of feeding, obliging these bats to socially learn via actual food cues such as sound (e.g., Page and Ryan 2006) or odor (e.g., Ratcliffe and ter Hofstede 2005; O’Mara et al. 2014).
In anthropogenically altered environments, flower-visiting bats can be observed to expand their ecological niche by incorporating novel food sources into their dietary repertoire. For example, G. soricina thrive in agricultural monocultures by exploiting flowers of old-world banana plants (Murphy et al. 2016; Alpízar et al. 2020), the usage of introduced ornamental plants may enable bats to occur in urban areas (Kruszynski et al. 2016; da Silva et al. 2017; Pellón et al. 2021) and groups of flower-visiting lesser long-nosed bats (Leptonycteris yerbabuenae) are only able to migrate through certain desert locations because they learned to utilize artificial hummingbird feeders at urban homes (Buechner and Sidner 2013). Such an adoption of novel food sources that are not part of the bats’ natural dietary repertoire can also be observed in species with different feeding habits. Insectivorous long-fingered bats (Myotis capaccinii) may have shifted in northern Israel from insectivory to semi-piscivory after a small fish species was introduced for mosquito control (Levin et al. 2006). Common vampire bats (Desmodus rotundus) parasitize livestock in rural areas (Bobrowiec et al. 2015) and on pets in urban areas (Rosa et al. 2013), while frugivorous bats may take advantage of cultivated crops (Parry-Jones and Augee 1991). However, there is no information on which degree the utilization of these food sources is driven by social learning, thus representing a socially transmitted behavior or in some regions even a local tradition, or whether it rather represents an opportunistic feeding behavior independently invented by multiple individuals (van Schaik 2010).
Progressively expanding their natural dietary repertoire with novel food sources is central for young bats during the transition from parental care to independent life, and a decisive role of social learning in this period is frequently suggested (Wright 2016; Rose et al. 2020). However, experiments on vertical social learning of foraging behavior in bats are scarce. Although the presence of experienced adults facilitated learning about foraging in young frugivorous and insectivorous bats (Wright et al. 2011; Ganesh et al. 2016), other studies on insectivorous and nectarivorous bats failed to provide evidence for a vertical transmission of foraging-related information from parents to offspring (Ripperger et al. 2019; Rose et al. 2019, 2020). For adult bats, several experiments in captivity demonstrated the application of social learning strategies when learning about novel food sources or dietary preferences (e.g., Ratcliffe and ter Hofstede 2005; Page and Ryan 2006; Wright et al. 2011; Jones et al. 2013; Ramakers et al. 2016), but comparable field experiments with free ranging bats are scarce (O’Mara et al. 2014) or rather focusing on learning about the spatial distribution of food (e.g., Barclay 1982; Wilkinson 1992; Ripperger et al. 2019; Rose et al. 2020). However, conclusions from mere lab or flight cage experiments have to be drawn carefully, since experiments in captivity often provide conditions that may rarely appear in nature, including forced spatial proximity of knowledgeable and learning individuals or a lack of alternative food sources. Therefore, an observed social transmission in artificial demonstrator-observer dyads may not be sufficient evidence for the respective transmission chains occurring also under natural conditions (Laland and Plotkin 1990). Further, all socially transmitted behaviors can be learned individually, as also focal bats in our control situation managed to feed from the novel food source, and every social transmission chain observed in animals was inevitably initiated at one point (Reader and Laland 2003). In this regard, it would be important to investigate the cues that facilitate an initial individual discovery of novel food sources in the wild. For instance, in the case of flower-visiting bats and hummingbird feeders, it seems conceivable that an initial discovery could be facilitated by the scent of fermenting sugar water, a scent bats may already be familiar with from natural nectar sources.
In conclusion, our study adds flower-visiting bats to the list of bats that are capable of using social information to learn about novel food sources, but relevance for and incidence in free-living bats remains unclear. Further studies should move from captivity to experiments with free ranging bats and investigate social learning about foraging behavior under natural conditions. Hereby, anthropogenically introduced food sources that are not part of the bats’ natural dietary repertoire may represent valuable study objects.
Data availability
The dataset collected and analyzed during the current study is available from the corresponding author on reasonable request.
References
Alpízar P, Schneider J, Tschapka M (2020) Bats and bananas: simplified diet of the nectar-feeding bat Glossophaga soricina (Phyllostomidae: Glossophaginae) foraging in Costa Rican banana plantations. Glob Ecol Conserv 24:e01254
Alvarez J, Willing M, Jones K, Webster D (1991) Glossophaga Soricina. Mamm Species 379:1–7
Amit R, Gordillo-Chávez EJ, Bone R (2013) Jaguar and puma attacks on livestock in Costa Rica. Hum Wildlife Interact 7:77–84
Aplin LM, Sheldon BC, Morand-Ferron J (2013) Milk bottles revisited: social learning and individual variation in the blue tit, Cyanistes caeruleus. Anim Behav 85:1225–1232
Aplin LM, Farine DR, Morand-Ferron J et al (2015) Experimentally induced innovations lead to persistent culture via conformity in wild birds. Nature 518:538–541
Barclay RMR (1982) Interindividual use of echolocation calls: eavesdropping by bats. Behav Ecol Sociobiol 10:271–275
Bobrowiec PED, Lemes MR, Gribel R (2015) Prey preference of the common vampire bat (Desmodus rotundus, Chiroptera) using molecular analysis. J Mammal 96:54–63
Buecher DC, Sidner R (2013) Long distance commutes by lesser long-nosed bats (Leptonycteris yerbabuenae) to visit residential hummingbird feeders. USDA Forest Service Proceedings RMRS-P-67:427–433
Clarin T, Borissov I, Page RA et al (2014) Social learning within and across species: information transfer in mouse-eared bats. Can J Zool 92:129–139
da Rocha PA, Ruiz-Esparza J, Ferrari SF (2018) Differences in the structure of the bat community between a cloud forest refuge and a surrounding semi-arid Caatinga scrubland in the northeastern Brazil. J Arid Environ 151:41–48
da Silva SSP, Guedes PG, Fagundes TMC, da Silva AF (2017) Observations of Pallas’s long-tongued bat, Glossophaga soricina (Pallas, 1766) (Chiroptera, Glossophaginae), visiting Dracaena reflexa Lam (Aspargaceae) flowers in an urban area of Rio de Janeiro (Brazil). Natureza Online 15:7–13
Fox J, Bouchet-Valat M (2017) Rcmdr: R Commander. R package version 2.4-1
Galef BG, Giraldeau L-A (2001) Social influences on foraging in vertebrates: causal mechanisms and adaptive functions. Anim Behav 61:3–15
Galef BG, Laland KN (2005) Social learning in animals: empirical studies and theoretical models. Bioscience 55:489
Ganesh A, Mukilan M, Marimuthu G, Rajan KE (2016) A novel food preference in the greater short-nosed fruit bat, Cynopterus sphinx: mother-pup interaction a strategy for learning. Acta Chiropterol 18:193–198
Giménez-Anaya A, Herrero J, Rosell C et al (2008) Food habits of wild boars (Sus scrofa) in Mediterranean coastal wetland. Wetlands 28:197–203
Hoppitt W, Laland KN (2013) Social learning: an introduction to mechanisms, methods and models. Princeton University Press, Princeton
Hörmann D, Tschapka M, Rose A, Knörnschild M (2021) Distress calls of nectarivorous bats (Glossophaga soricina) encode individual and species identity. Bioacoustics 30:253–271
Jones PL, Ryan MJ, Flores V, Page RA (2013) When to approach novel prey cues? Social learning strategies in frog-eating bats. Proc R Soc B 280:20132330
Kottsieper J, Schwemmer P, Markones N et al (2019) An invasive alien bivalve apparently provides a novel food source for moulting and wintering benthic feeding sea ducks. Helgol Mar Res 73:11
Kruszynski C, Diniz-Reis TR, Pedrozo AR (2016) A new food resource for Glossophaga soricina (Mammalia: Chiroptera) in southeast Brazil. Bol Da Soc Bras Mastozool 77:124–130
Laland KN, Plotkin HC (1990) Social learning and social transmission of foraging information in Norway rats (Rattus norvegicus). Anim Learn Behav 18:246–251
Laland KN, Atton N, Webster MM (2011) From fish to fashion: experimental and theoretical insights into the evolution of culture. Philos Trans R Soc B 366:958–968
Levin E, Barnea A, Yovel Y, Yom-Tov Y (2006) Have introduced fish initiated piscivory among the long-fingered bat? Mamm Biol 71:139–143
Maguiña R, Muchhala N (2017) Do artificial nectar feeders affect bat-plant interactions in an Ecuadorian cloud forest? Biotropica 49:586–592
Murphy M, Clare EL, Rydell J et al (2016) Opportunistic use of banana flower bracts by Glossophaga soricina. Acta Chiropterol 18:209–213
O’Mara MT, Dechmann DKN, Page RA (2014) Frugivorous bats evaluate the quality of social information when choosing novel foods. Behav Ecol 25:1233–1239
Page RA, Ryan MJ (2006) Social transmission of novel foraging behavior in bats: frog calls and their referents. Curr Biol 16:1201–1205
Parry-Jones K, Augee ML (1991) Food selection by grey-headed flying foxes (Pteropus poliocephalus) occupying a summer colony site near Gosford, New South Wales. Wildl Res 18:111–124
Pellón JJ, Mendoza JL, Quispe-Hure O et al (2021) Exotic cultivated plants in the diet of the nectar-feeding bat Glossophaga soricina (Phyllostomidae: Glossophaginae) in the city of Lima, Peru. Acta Chiropterol 23:107–117
Prat Y, Yovel Y (2020) Decision making in foraging bats. Curr Opin Neurobiol 60:169–175
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Ramakers JJC, Dechmann DKN, Page RA, O’Mara MT (2016) Frugivorous bats prefer information from novel social partners. Anim Behav 116:83–87
Ratcliffe JM, ter Hofstede HM (2005) Roosts as information centres: social learning of food preferences in bats. Biol Lett 1:72–74
Reader SM, Laland KN (2003) Animal innovation: an introduction. Animal innovation. Oxford University Press, Oxford, pp 3–35
Ripperger S, Günther L, Wieser H et al (2019) Proximity sensors on common noctule bats reveal evidence that mothers guide juveniles to roosts but not food. Biol Lett 15:20180884
Rosa A, de Almeida M, Martorelli L, et al. (2013) Attack of Desmodus rotundus (Phyllostomidae) on urban dogs from Sao Paulo City, Southeastern Brazil. Poster at 16th International Bat Research Conference, San Jose, Costa Rica
Rose A, Kolar M, Tschapka M, Knörnschild M (2016) Learning where to feed: the use of social information in flower-visiting Pallas’ long-tongued bats (Glossophaga soricina). Anim Cogn 19:251–262
Rose A, Wöhl S, Bechler J et al (2019) Maternal mouth-to-mouth feeding behaviour in flower-visiting bats, but no experimental evidence for transmitted dietary preferences. Behav Process 165:29–35
Rose A, Tschapka M, Knörnschild M (2020) Visits at artificial RFID flowers demonstrate that juvenile flower-visiting bats perform foraging flights apart from their mothers. Mamm Biol 100:463–471
Thiele J, Winter Y (2005) Hierarchical strategy for relocating food targets in flower bats: spatial memory versus cue-directed search. Anim Behav 69:315–327
Timm RM, LaVal RK (1998) A field key to the bats of Costa Rica. Occas Publ Ser Cent Lat Am Stud 22:1–30
Tryjanowski P, Morelli F, Skorka P et al (2015) Who started first? Bird species visiting novel birdfeeders. Sci Rep 5:6–11
Tschapka M, Dressler S (2002) Chiropterophily: on bat-flowers and flower-bats. Curtis Bot Mag 19:114–125
van Schaik CP (2010) Social learning and culture in animals. In: Kappeler P (ed) Animal behaviour: evolution and mechanisms. Springer, Heidelberg, pp 623–653
von Helversen O (1995) Blumenfledermäuse und Fledermausblumen—Wechselbeziehungen zwischen Blüte und Bestäuber und energetische Grenzbedingungen. Rundgespräche Der Kommission Für Ökologie 10:217–229
von Helversen D (2004) Object classification by echolocation in nectar feeding bats: size-independent generalization of shape. J Comp Physiol A 190:515–521
Weissgerber TL, Milic NM, Winham SJ, Garovic VD (2015) Beyond bar and line graphs: time for a new data presentation paradigm. PLOS Biol 13:e1002128
Wilkinson GS (1992) Information transfer at evening bat colonies. Anim Behav 44:501–518
Wilkinson GS, Boughman J (1999) Social influences on foraging in bats. Mammalian social learning: comparative and ecological perspectives. Cambridge University Press, Cambridge, pp 189–204
Wright GS (2016) Social learning and information transfer in bats: conspecific influence regarding roosts, calls, and food. Sociality in bats. Springer International Publishing, Berlin, pp 211–230
Wright GS, Wilkinson GS, Moss CF (2011) Social learning of a novel foraging task by big brown bats, Eptesicus fuscus. Anim Behav 82:1075–1083
Wright GS, Wilkinson GS, Moss CF (2020) Social facilitation in short-tailed fruit bats, Carollia perspicillata (Linnaeus). Behaviour 157:1193–1210
Zajonc RB (1965) Social facilitation. Science 149:269–274
Acknowledgements
We are grateful to the Costa Rican authorities for allocating research permits (Nº ACG-PI-057-2014 and ACG-PI-059-2015). We thank Roger Blanco and all the other people from the Área de Conservación Guanacaste (ACG) and the Parque Nacional Santa Rosa for excellent logistic support and providing infrastructure for fieldwork. We further thank two anonymous reviewers for their constructive comments on the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was funded by a stipend from the Rosa Luxemburg Foundation to AR and a Heisenberg Fellowship of the Deutsche Forschungsgemeinschaft (DFG; German Research Foundation) to MK (DFG KN935 3-1).
Author information
Authors and Affiliations
Contributions
All authors contributed to the implementation of the study. AR designed the experiment, collected and analyzed the data and wrote the first draft of the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed. Handling of animals was reduced to the necessary minimum and handling was always performed with respect to the avoidance of stress. Permissions for the work in Costa Rica were granted by the Costa Rican government (permit numbers: ACG-PI-057-2014 and ACG-PI-059-2015).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 (AVI 4796 KB)
Supplementary file3 (AVI 7047 KB)
Supplementary file4 (AVI 5117 KB)
Supplementary file5 (AVI 2556 KB)
Supplementary file6 (AVI 7694 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rose, A., Tschapka, M. & Knörnschild, M. Social information facilitates learning about novel food sources in adult flower-visiting bats. Anim Cogn 26, 1635–1642 (2023). https://doi.org/10.1007/s10071-023-01807-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-023-01807-9