Abstract
Dogs excel at understanding human social-communicative gestures like points and can distinguish between human informants who vary in characteristics such as knowledge or familiarity. This study explores if dogs, like human children, can use human social informants’ past accuracy when deciding whom to trust. Experiment 1 tested whether dogs would behave differently in the presence of an accurate (vs. inaccurate) informant. Dogs followed an accurate informant’s point significantly above chance. Further, when presented with an inaccurate point, dogs were more likely to ignore it and choose the correct location. Experiment 2 tested whether dogs could use informant past accuracy to selectively follow the point of the previously accurate informant. In test trials when informants simultaneously pointed at different locations (only one of which contained a treat), dogs chose the accurate informant at chance levels. Experiment 3 controlled for non-social task demands (e.g. understanding of hidden baiting and occlusion events) that may have influenced Experiment 2 performance. In test trials, dogs chose to follow the accurate (vs. inaccurate) informant. This suggests that like children, dogs may be able to use informants’ past accuracy when choosing between information sources.
Similar content being viewed by others
Introduction
Whether deciding whom to play with or which couch to search under to find a lost ball, social animals such as dogs and humans must often make decisions in the absence of their own information, instead relying on information presented by their social partners. Engaging in solitary learning in a novel situation can often be inefficient or even impossible, so it can be beneficial for social animals to follow the information presented by others. However, this often requires reconciling conflicting information from multiple social partners, such as having to choose between two possible new foraging locations each of which has been chosen or endorsed by others. When deciding whose information to rely on in these conflicts, social learners that are selective, rather than indiscriminate, in their trust are at an advantage (e.g. Harris et al. 2018; Laland 2004). Being able to selectively trust an individual on the basis of their knowledge, confidence or accuracy can help social learners avoid making costly mistakes, ranging in severity from time wasted going in the wrong direction, to a dangerous predator ignored. In particular, it may be highly advantageous to track informants’ past accuracy (Harris et al. 2018; Poulin-Dubois and Brosseau-Liard 2016). Social informants who were previously helpful and gave good information in the past are likely well-informed and trustworthy and will continue to give good information. Informants who were previously wrong, either due to lack of knowledge or bad intentions should perhaps not be trusted in the future (Mills 2013). In this set of studies, we explored whether domestic dogs are able to use informant past accuracy to choose between two individuals.
Selective social learning
Humans are social animals, and from a young age we are highly adept at using others’ information when we do not know the answer for ourselves. By around 4 years of age, children are proficient at using prior accuracy as a cue for choosing between informants (for a review see Harris et al. 2018 or Poulin-Dubois and Brosseau-Liard 2016). Three-year-olds also appear to monitor informants for inaccuracy, and selectively trust a previously accurate (vs. a previously inaccurate) informant, however, they have trouble differentiating between an accurate and neutral informant (Corriveau et al. 2009). By the time children are four-years-old they are also able to ignore inaccurate testimony in favour of their own information about the location of a hidden object (Ma and Ganea 2010; Ganea et al. 2011). Moreover, by four years of age, children can evaluate accuracy probabilistically and choose between informants who have a mixed history of accuracy, for instance trusting a previously more accurate informant over a previously less accurate one if the accurate informant was more correct in the past (i.e. 75% correct vs 25% correct; Pasquini et al. 2007).
Children’s demonstrated ability to selectively follow and trust accurate informants as well as to ignore inaccurate informants shows us that children are able to critically evaluate their social partners. This relatively complex process involves tracking the current reliability of information as well as remembering past information and continually updating trust (or distrust) in others. Like children, great apes display selective trust with conspecifics in versions of the human trust task, flexibly adjusting their behavior depending on their partner’s level of reciprocity or trustworthiness (e.g., Engelmann and Herrmann 2016; Engelmann et al. 2015). Great apes also demonstrate some levels of selective trust with humans on the basis of prior reliability (Schmid et al. 2017). Tracking informant accuracy and knowledge provides many benefits, but selective trust on the basis of prior accuracy also requires a high level of social cognitive skills. Do other social animals also demonstrate this ability, or is it a socio-cognitive skill that is unique to primates (and perhaps even to great apes)?
Domestic dogs provide an excellent model for studying the cognitive mechanisms and evolutionary origins of social cognition in general and selective trust in particular, for several reasons. Like wolves and chimpanzees, the ancestors of domestic dogs were cursorial social hunters, meaning that they historically needed to work in close coordination with others in a relatively organized way (Lea and Osthaus 2018). In addition, dogs have an arguably unique evolutionary history that is closely entwined with ours. Perhaps as a result of their domestication and their evolutionary niche living in a human environment and partnering with humans, they often outperform our genetically closer ape relatives at using human social-communicative cues, which may reflect more human-like social-cognitive abilities (Hare et al. 2002) or more attunement to human (or human-like) communicative cues (Bräuer et al. 2006).
Like human children and apes, dogs have demonstrated some ability to evaluate the reliability of their social partners. For instance, dogs can make reputation-like inferences about an individual on the basis of that individual’s treatment of others. Dogs preferentially approached a novel demonstrator whom they had observed being generous to an experimenter, as compared to a demonstrator who was observed to withhold food from the experimenter (Kundey et al. 2011). Similarly, highly trained agility dogs preferred prosocial (helpful to an experimenter) over antisocial (hindering the experimenter) demonstrators, though this ability may not generalize (Nitzschner et al. 2014; Silver et al. 2020).
Dogs can also differentiate knowledgeable versus ignorant social informants. In paradigms known as guesser-knower tasks (adapted from the literature on primate social learning, e.g., Povinelli et al. 1990), a neutral individual first shows the dog a treat, and then hides it out of the dogs’ view. One informant watches as the treat is hidden, while a second informant is not able to see the baiting. In these studies, dogs, like chimpanzees, choose the location indicated by the knowledgeable informant, suggesting that they can distinguish someone who is guessing from someone who knows the location of the hidden food (Catala et al. 2017; Maginnity and Grace 2014).
Dogs are also able to learn selectively from others on the basis of characteristics beyond others’ knowledge or ignorance. Dogs’ preferentially follow social cues from their owners as opposed to an unfamiliar experimenter to find hidden food (Cook et al. 2014). Together, this suggests that, at least in some situations, dogs (like children and non-human great apes) are able to discriminate between social informants and selectively trust one over another to make the most advantageous choice. This makes them promising candidates for exploring the tracking and comparison of informant accuracy.
Dogs’ understanding of the pointing gesture
Dogs’ ability to follow human communicative cues, particularly gaze and pointing, has also been widely studied (for a review see Kaminski and Nitzchner 2013). These studies often use object choice tasks and ask dogs to follow an (accurate) human gesture, such as gazing or pointing, in order to correctly choose among a series of identical containers to find a (either hidden or visible) food item. Dogs are particularly adept at following human points compared to apes and other primates (Bräuer et al. 2006) and can follow pointing gestures from puppyhood without explicit training (Agnetta et al. 2000; Hare et al. 2002; Hare and Tomasello 1999; Lakatos et al. 2012).
Given dogs’ success at following human pointing gestures, particularly relative to other non-human species, there has been some controversy about precisely how dogs interpret human pointing. Some researchers have suggested that dogs perceive human points as informative social cues, (Scheider et al. 2011) meaning that they interpret points as conveying information. Others argue that dogs do not understand human points as informative communicate cues, but instead obligately follow points because they interpret them as an imperative or a command (Kaminski et al. 2012; Topál et al. 2009). Still others suggest that dogs learn to follow the pointing gesture through gradual associative learning or local enhancement, either over the course of their life or even over the course of the study, and follow points because they have learned to attend to the object at the end of the point as likely to be rewarding, and not because they perceive points communicatively (Udell et al. 2008; Wynne et al. 2008).
Low-level explanations for dogs’ skillful following of human points (e.g., local enhancement or association built over trials) have been largely ruled out as they do not explain dogs’ use of subtler cues like head orientation, eye gaze, and more complex pointing gestures like cross-body momentary points (see results from Agnetta et al. 2000; McKinley and Sambrook 2000; Miklósi and Soproni 2006). Low-level explanations also do not explain dogs’ sensitivity to the context of point delivery, namely that they follow points directed at them, but not unintended movements that looked very similar to the intentional point (Kaminski et al. 2012). There is also some evidence that dogs do not respond to pointing as a command, as dogs follow and ignore pointers on the basis of their accuracy, irrespective of their authority levels. This uniform response to authority in pointing is a marked difference from their response trained commands like sitting (where they obeyed the high-authority figure more) (Scheider et al. 2013).
In this context, dogs’ response to inaccurate pointing may be particularly relevant to understanding whether they respond to pointing as a command, in which case they should follow even consistently inaccurate pointers, or see pointing as an informative social cue, in which case they should follow their own accurate information (i.e., ignore the inaccurate pointer). So far, the evidence on whether dogs are sensitive to inaccurate information, and inaccurate pointing in particular, has been mixed. Many studies have suggested that dogs respond differently to accurate and inaccurate points (Kundey et al. 2010; Petter et al. 2009; Takaoka et al. 2015). For example, when dogs interacted separately with two human informants, one of whom consistently pointed towards the correct location of a piece of hidden food and the other to the incorrect location, dogs selectively approached the accurate pointer’s location and approached the inaccurate pointer’s location at chance levels (Petter et al. 2009). However, dogs sometimes persist in approaching the location indicated by the inaccurate person, leading some researchers to suggest that dogs are unable to ignore an inaccurate point, supporting an interpretation of pointing as a command, rather than a communicative social cue (Dwyer and Cole 2018). However, the differing performance between accurate and inaccurate pointers across most studies supports the notion that dogs interpret pointing as a communicative cue, or at most a moderate imperative that can be disregarded when better information is available.
Dogs are able to respond differently to accurate and inaccurate pointers by relying on their own experiential knowledge, which may act as a mediating factor on their point following behavior by out weighing the social information presented. Over the course of a block of trials where the treat was visible at all times, including at the time the choice was made, dogs learned to ignore an inaccurate informant who pointed at the empty location (Kundey et al. 2010). In addition, unlike paradigms used in primate studies where subjects may complete hundreds of trials to learn about cues predicting informant accuracy (e.g. Povinelli et al. 1990), dogs are able to learn to ignore inaccurate points in a relatively short amount of time (12 trials in Kundey et al. 2010). Further, dogs appear to flexibly adapt their behavior to the changing accuracy of a human informant in a two-object choice task. Without observing where the food was hidden, dogs saw an informant point to the baited location, providing evidence that the informant was accurate. After two trials, the informant first showed the dog the contents of both locations (baited and un-baited) and then began pointing to the un-baited location, now becoming an inaccurate informant. Dogs now had to ignore the point of the previously accurate informant and follow their own visual information to find the food. Finally, baiting was hidden again, and the informant resumed pointing accurately. Despite the point now being correct, on the basis of their past experience dogs were less likely to follow the point than in the first phase. Together this suggests that dogs are able to incorporate their recent past experience with informants to change their behavior (Takaoka et al. 2015).
Across studies, seeing the location of the treat (either during baiting or throughout the trial) appears to be important in enabling dogs to ignore inaccurate human points, perhaps by providing an alternative reliable cue to follow, as well as strong evidence that the pointer is inaccurate. Dogs do not ignore an inaccurate point if it is the only cue available to them, which occurs when the location of the treat is never revealed (Petter et al. 2009; Kundey et al. 2011). However, like 4-year-old children, they can ignore an inaccurate human informant in favor of their own information if they know the location of hidden food—i.e., they rely on their current or prior visual experience (Scheider et al. 2013). In fact, if dogs visually observe the hiding of a treat, followed by an inaccurate point, they are more successful at ignoring the inaccurate point and choosing the correct location as compared to when only olfactory cues from the treat and an inaccurate point are presented (Szetei et al. 2003).
Overall, previous findings suggest that dogs are very successful at following accurate points and have some success in ignoring inaccurate points. They have also been successful at differentiating between informants on other characteristics like knowledge and familiarity (Catala et al. 2017; Kundey et al. 2011; Maginnity and Grace 2014; Cook et al. 2014). However, it is not presently known whether, in the absence of personal information (e.g., observing where the treat is currently located) dogs, like human children, can use past accuracy to choose between informants. Exploring the possible existence of this shared ability will shed light on the way that dogs learn from humans, and on their social learning abilities more generally. Further, by exploring whether dogs are able to critically evaluate their social informants, we can learn more about the evolutionary pressures that led to the development of selective social learning abilities. We can also learn about the extent to which these abilities are broadly shared or unique to humans (and perhaps other primates). In the current set of studies, we aimed to investigate whether dogs can evaluate informants based on their past accuracy, and selectively follow an accurate informant (vs. an inaccurate informant), in order to find hidden treats.
The current study
In Experiment 1, we first investigated dogs’ ability to integrate their own visual observations about treat location with accurate or inaccurate social cues, using an object choice task. This integration is a prerequisite to selective trust based on accuracy, as if dogs do not perform differently when presented with a currently accurate informant than when presented with a currently inaccurate informant, then they may not be sensitive to or able to track an informant’s present accuracy, and so it is unlikely that they would be able to compare informants based on their relative past accuracy, when the two informants conflict in the present.
In Experiment 2, we explored whether dogs would use informants’ prior accuracy as a cue for where to search for the treat. Given that dogs responded differently to the accurate and inaccurate informants in Experiment 1, we wanted to see if dogs could capitalize on this differentiation to selectively choose the location indicated by the previously accurate pointer. In Experiment 3, we aimed to rule out possible non-social reasons for dogs’ poor performance when presented with the simultaneous conflicting informants in Experiment 2. In particular, we ruled out potential difficulty understanding the two alternative choice search task when baiting was hidden, difficulty understanding occlusion events, and difficulty tracking two simultaneously pointing informants.
All three experiments were approved by the University of Toronto’s University Animal Care Committee (UACC). Procedures across all three experiments were in accordance with Ontario’s Animals for Research Act, the federal Canadian Council on Animal Care and fully complied with the APA Ethical Standards for Use of Animals in Research.
Experiment 1
In Experiment 1, we aimed to clarify past results and determine whether dogs discriminate an accurate from an inaccurate human informant, and whether they interpret points as imperative commands. Dogs either interacted with an accurate informant or an inaccurate informant in a between-subjects design. Previous results have indicated that dogs excel at following accurate pointing and behave differently in the presence of accurate and inaccurate pointers, especially when the location of the food is known (Dwyer and Cole 2018; Kundey et al. 2010; Petter et al. 2009; Takaoka et al. 2015). Given this, we hypothesized that if dogs interpret points as commands and obligately follow them, they should be equally likely to follow both informants, leading them to succeed at finding the treat in the presence of the accurate pointer and fail (be below chance) in the presence of the inaccurate pointer. If dogs only consider their own visual information, they should be equally successful in the presence of both informants, since they always observe the treat being hidden. Finally, if dogs are sensitive to the conflict between their own visual information and the communicative point of the inaccurate informant, they should follow the point less than when presented with an accurate informant, but may still be less successful at correctly locating the treat than in the presence of the accurate informant.
Methods
Participants
The participants were 43 pet dogs, of which 35 were included in analyses (male = 20; mean age = 50.59 months; age range = 8.54–146.69 months). All participants were recruited on a volunteer basis from the Greater Toronto Area and there were no breed requirements. Dogs were randomly assigned to one of two conditions: The Accurate condition (n = 16) or the Inaccurate condition (n = 19).Footnote 1
A total of eight dogs were excluded, four from the Inaccurate condition, and 4 from the Accurate condition, based on criteria determined prior to the start of data collection: (1) failing a pre-screening object permanence task (n = 5), (2) study noncompletion due to anxiety or lack of response (n = 3). No dogs were excluded for displaying a significant side bias, defined as 12/12 searches to the same side in the final task, the Informant phase.Footnote 2 See supplementary materials for a detailed breakdown of phases and reasons for exclusion for all excluded dogs.
Materials and Procedure
This experiment took place in three phases: the warm-up phase, the object permanence phase, and the informant phase. All three phases involved searching for a hidden treat under one of two 16 oz plastic cups, both of which were false baited with additional inaccessible treats to control for olfactory cues. All phases were videotaped from two angles, one approximating the dog’s perspective and one with a full room view. Owners were in the room for the duration of the session in accordance with ethics requirements but were seated behind and out of view of the dog and were instructed to not intervene.
Each session involved three trained research assistants, one Handler, one Neutral Experimenter, and one Informant. The Handler ensured the dog was sitting/waiting at the start line prior to each trial and maintained their position to the side of and slightly behind the dog. The Neutral Experimenter carried out all experimental procedures described below for the warm-up and object permanence phases. The Informant was a female research assistant the dog had not previously met and carried out the procedure described below for the informant phase.
The warm-up phase ensured that dogs were familiar with and comfortable retrieving a treat hidden under a cup. The Neutral Experimenter sat on the ground facing the dog, showed a treat reward to the participant, placed it on the ground and then covered it with a cup. Then the Handler verbally released the dog and released any tension in the leash and the Neutral Experimenter recorded whether the dog successfully knocked the cup over to get the treat. This sequence was repeated until the participant successfully knocked over the cup on three consecutive trials, or they reached the maximum of 15 repetitions (not observed in our sample).
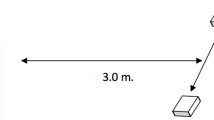
The object permanence phase was used to familiarize dogs with the task of choosing between two search locations to locate a treat that they watched being hidden, in the absence of any social cues, and to establish whether the dog could flexibly switch between the two search locations. Two cups were placed 0.5 m apart from each other and 1.2 m from the dog’s start line (see Fig. 1). The Neutral Experimenter sat behind the cups facing the dog. The Neutral Experimenter then made eye-contact with the dog and presented a high-value treat to the dog between the thumb and index finger of one hand and placed it under one of the cups which was lifted by the Experimenter’s other hand. The Neutral Experimenter then lifted the other cup to show there was nothing underneath. The presentation order (treat first or empty cup first) was counterbalanced.
After the presentation, the Neutral Experimenter looked down at the ground in front of them, between the two search locations. The Handler, who was standing to the side and slightly behind the dog looking down and not observing the demonstration, then verbally released the dog and released any tension in the leash. The Neutral Experimenter then recorded which cup the dog first searched as well as which cup, they first made contact with, which counted as a choice. In order to demonstrate they could flexibly locate the hidden treat in both locations, dogs were required to choose the cup covering the treat four times in a row within a maximum of eight trials. Dogs were given what was under the selected cup (either a treat or nothing) and were shown what was under the unselected cup but were not permitted to interact with it (i.e. eat the treat).
In the informant phase, participants met either the Accurate, or Inaccurate Informant with the same starting position as in the object permanence phase (see Fig. 1). The Informant (accurate or inaccurate) wore a black or white t-shirt and took a kneeling or seated position on the floor behind the cups, facing the dog. With the dog watching, the Informant executed the same baiting procedure as in the object permanence phase. The informant then performed a dynamic proximal point (finger approximately 10 cm from the cup) using the same-sided arm (left arm to left cup) to one of the cups while also gazing at that cup. This style of pointing was chosen as past research has suggested it is the most attention getting and easy for dogs to follow (Miklósi and Soproni 2006). The Accurate Informant always pointed to the cup that concealed the treat, and the Inaccurate Informant always pointed to the empty cup. Following a brief waiting period of 2–3 s, the Handler verbally released the participant and any tension in the leash and the Informant recorded where the dog searched. Dogs participated in 12 trials. In the object permanence and informant trials the location of the treat was pseudo-randomized between the left and right cups across trials, ensuring the treat was placed in both locations an equal number of trials, and did not appear under the same cup more than twice in a row.Footnote 3 Trials where the dog did not make a choice within 30 s of being released were coded as “No Response” and the trial was repeated.
Data scoring and analysis
Across trials, a dog’s choice was considered to be physical contact with one of the two cups. A trial was considered correct if the dog chose the location where the treat was hidden. For dogs in the Accurate condition, the number of correct trials was identical to the number of point-following trials, as the Accurate Informant always pointed to the location of the hidden treat. For dogs in the Inaccurate condition, the number of correct trials was the opposite as the number of point-following trials, as dogs had to not follow the point to get the treat. Live coding was performed by the Neutral Experimenter and Informant, and an additional coder who was blind to the purpose of the study and the study’s hypothesis also coded for choice for 25% of the videos to ensure that the data was recorded accurately. Initial recoded reliability was very high at 99.39%. Trials in which the subject did not make a choice within 30 s or where the experimenter made an error were repeated and the original trial was excluded from analysis. Statistical analyses were conducted using R statistical software version 3.5.2 (R Development Core Team 2013).
Results and discussion
Overall, dogs performed very well on the warm-up and object permanence trials of the experiment. In the warm-up trials, dogs successfully met the criteria of finding the hidden treat under the single cup three times (M# trials to criterion = 3.79, S.E. = 0.21). The object permanence trials established dogs’ ability to track hidden objects and switch search locations flexibly to find the treat, which was a prerequisite to the informant trials. Dogs successfully found the treat four times in a row (M# trials to criterion = 5.06, S.E. = 0.39).
In the informant trials of the Accurate condition, dogs were significantly more likely to choose the correct cup (the one containing the treat) than expected by chance (chance = 6/12 correct choices, M = 11.63/12, S.E. = 0.20), t(15) = 27.91, p < 0.001. There was no strong evidence of learning over the course of trials as dogs were already close to ceiling on trial 1, with 15/16 dogs successfully locating the hidden treat, and similarly 15/16 dogs successfully located the hidden treat on trial 12. These results align with previous research showing that dogs excel at finding hidden treats in the presence of an accurate pointer and are able to do so without training (Petter et al. 2009; Kundey et al. 2010; Takaoka et al. 2015).
Dogs also succeeded in ignoring the inaccurate pointer during the informant trials to locate the hidden treat (M = 7.89/12, S.E. = 0.58), t(18) = 3.28, p = 0.004. Over the course of 12 trials, dogs got marginally worse at finding the hidden treat with 15/19 dogs successfully ignoring the inaccurate point in favor of their own information on the first trial and only 10/19 dogs ignoring the inaccurate pointer on trial 12. Though fewer dogs located the treat on trial 12 (vs. trial 1), a general linear model revealed that trial number was not a significant predictor of performance, Β = − 0.01, S.E. = 0.009, t = − 1.33, p = 0.18. Dogs’ ability to ignore an inaccurate point cue to obtain a treat suggests that they can assess the relative usefulness of the point cue, given the other information that is available to them (i.e., their prior visual experience), and flexibly alter their behavior accordingly.
A t test for independent samples showed that dogs were significantly more successful at finding the hidden treat in the Accurate condition than in the Inaccurate condition, t(22.27) = 6.105, p < 0.001. Using point following rather than correct search as a metric (see Fig. 2), dogs were more likely to follow the Accurate Informant’s point (M = 11.63/12, S.E. = 0.20) than the Inaccurate Informant’s point (M = 4.11/12, S.E. = 0.58), t(22.27) = 12.31, p < 0.001.
This difference indicates that not only are dogs successful at using their own knowledge to locate the hidden treat, but they differentiate between the Accurate and Inaccurate Informants, indicated by their different performance across the two conditions. These results also suggest that dogs are more successful at finding a reward when their previous observation about its location corresponds with the social cue provided by the informant, compared with when these sources of information are in conflict. Given that dogs respond differently to accurate and inaccurate points, we can now ask whether they can use the previous accuracy of two informants to evaluate the quality of the information they are providing, and choose directly between them when the location of the treat is unknown, and the informants’ points are in conflict.
Experiment 2
Given that dogs are sensitive to a single informant’s inaccuracy in the present moment, we wanted to see if dogs can use previous experience with informants to choose between them. In Experiment 2 we used a within-subjects design to see if dogs can track and use their previous experience with the accuracy of two informants to choose between them when they provide conflicting information. Experiment 2 was closely modeled on similar studies with young children (e.g., Corriveau et al. 2009; Pasquini et al. 2007) in which the participants are first given experience with two informants' (present) accuracy in a situation where the participant also knows the correct answer, and then given a choice between information from the previously accurate and inaccurate informants in a situation where the participant does not know the answer (and the informants are in conflict). As in Experiment 1, when interacting with an individual informant dogs had information from their own observation of where the treat was hidden, in addition to information from the informant’s point. After experiencing both informants separately, dogs were presented with both informants providing conflicting information where the only cue available was dogs’ experience of the informants’ previous accuracy. We hypothesized that if dogs, like children, can monitor informants’ past accuracy and use that information to make choices, that they would be more likely to follow the point of the previously accurate informant over the previously inaccurate informant in the test trials.
Methods
Participants
The participants were 28 pet dogs, of which 16 were included in analyses (male = 7; mean age = 48.25 months; range = 23–99 months).Footnote 4 All participants were recruited on a volunteer basis from the Greater Toronto Area and there were no breed requirements. A power analysis was conducted in the same manner as in Experiment 1, revealing sufficient power with this sample size to detect medium-to-large effects. Twelve dogs were excluded based on criteria determined prior to the start of data collection from the study, (1) if they displayed a significant side bias on the test trials, defined as 12/12 to the same side (n = 3), (2) study noncompletion due to anxiety or lack of interest (n = 5), (3) significant errors made by the owner-handler (n = 1). Three dogs were excluded due to climate control issues in the test room, namely excessive heat leading to unsuitable conditions for dogs. See supplementary materials for a detailed breakdown of phases and reasons for exclusion.
Materials and procedure
All the materials used were the same as Experiment 1 with the addition of a 2-piece opaque acrylic occluder to hide the baiting procedure in the final phase (conflicting informant test trials). Experiment 2 took place in four phases; warm-up, first informant history, second informant history, and conflicting informant test trials. Due to the increased length of the task, object permanence (from Experiment 1) was omitted. As two informants would now be present during the conflicting informant test trials, to minimize the number of people present in the room, handling was done by the owner seated in a chair behind the start line across all phases. Owners received both online and in-person training prior to the test session to minimize the likelihood of them providing cues to their dog and had their eyes closed during the baiting procedure. In addition to the owner handling, three people were involved in each session. The Neutral Experimenter carried out the warm-up and provided the owner with instructions and information as needed. Two informants (both females who the dog had not previously interacted with) carried out their respective histories individually, and both were present for the conflicting informant test trials. The Neutral Experimenter also acted as the coder across phases.
The procedure of the warm-up was identical to Experiment 1. During the informant histories trials, dogs met the Accurate and Inaccurate informants individually (order counterbalanced across dogs), and informants wore either a black or white T-shirt (order counterbalanced) to help dogs distinguish between them visually. As in Experiment 1, during the informant histories trials, informants held up a high value treat in one hand, held between the thumb and index finger, and showed that their other hand was empty. Then the informant simultaneously lowered both hands, placing the treat on the ground in front of one of the cups, and holding her empty hand in front of the other. She then used both hands to simultaneously move both cups to cover the ground immediately in front of them, covering the treat and the empty locations. After the treat and empty location were covered, she performed a dynamic point identical to that in Experiment 1 to either the cup with the treat (Accurate informant cue) or to the empty cup (Inaccurate informant cue).
In the conflicting informants test trials, both informants were seated 0.75 m apart facing the dog at the start line (see Fig. 3). Trials began with informants each moving their half of the occluder into position blocking the dog’s view of the search locations. With the occluders hiding their actions, the accurate informant placed a treat under their cup and the inaccurate informant identically mirrored the movements but did not place a treat. Then the informants removed the occluders revealing the two cups to the dog. The informants both performed a dynamic proximal point at their respective cups while simultaneously gazing at the cup and maintained this position until the dog made a choice. This procedure was repeated for a total of 12 trials, with the treat being placed equally to the right and left according to a pseudo-randomized sequence. To avoid informants pointing over one another and asymmetrical points (where the informant is at/on the non-target location and points to the distant target), which may be harder for dogs to track, the informants switched sides so that the accurate informant was always directly behind the baited cup (Miklósi and Soproni 2006).
Data scoring and analysis
Choice and accuracy criteria were the same as in Experiment 1. In the presence of the Accurate Informant, the number of correct trials was identical to the number of point-following trials, as the Accurate Informant always pointed to the location of the hidden treat. In the presence of the Inaccurate Informant, the number of correct trials was the opposite as the number of point-following trials, as dogs had to not ignore the pointer to get the treat. In conflicting informants test trials, no value was assigned for point following as two points were presented. An additional coder who was blind to the purpose of the study’s hypothesis also coded for choice in 25% of videos to ensure that the data was recorded accurately. Recoded reliability was very high at 99.31%. As in Experiment 1, trials in which the subject did not make a choice within 30 s or where the experimenter made an error were repeated and the original trial was excluded from analysis. As in Experiment 1, Statistical analyses were conducted using R statistical software version 3.5.2 (R Development Core Team 2013).
Results and Discussion
As in Experiment 1, in the History trials of Experiment 2, dogs were successful at locating the hidden treat in the presence of the accurate informant (M = 9.88, S.E. = 0.50), t(15) = 7.77, p < 0.001. In the presence of only the Accurate informant 14/16 dogs successfully located the treat on the first trial, and 13/16 dogs successfully located the treat on trial 12. As in Experiment 1, there was no evidence of learning across the trials with Accurate informant, Β = 0.007, S.E. = 0.0086, t = 0.84, p = 0.41. Critically, as in Experiment 1, dogs followed the inaccurate informant’s points (see Fig. 4) significantly less than the accurate informants’ (M = 5.69, S.E. = 0.68), t(15) = 5.51, p < 0.001. These results are in keeping with the findings from Experiment 1 that dogs behave differently in the presence of an accurate and an inaccurate pointer.
However, unlike in Experiment 1, only 7/16 dogs found the hidden treat on the first trial in the presence of the inaccurate informant, and dogs did not find the hidden treat more often than expected by chance over the trials (M = 6.31, S.E. = 0.68), t(15) = 0.36, p = 0.72. On trial 12, 10/16 dogs successfully located the hidden treat, but a linear model revealed that there was no evidence of learning across trials, Β = 0.019, S.E. = 0.01, t = 1.93, p = 0.07. This failure to find the treat at above-chance levels may be due to the removal of the object-permanence task that was used in Experiment 1, which may have familiarized dogs with the asocial task components in the previous Experiment. It is also possible that evaluating two informants sequentially is more challenging for dogs. Finally, it is possible that consistently ignoring an inaccurate point in favor of their own information is difficult for dogs, and that the ability to disregard inaccurate points is a capability of the species but may not be generalizable to all dogs. Nevertheless, dogs’ consistently following the accurate pointer, while not consistently following the inaccurate one, in a within subjects task, suggests that they do differentiate the two informant types, and do not obligately follow pointing gestures.
Despite the fact that dogs differentiated between the accurate and inaccurate informants during the history trials, dogs chose between the informants at chance-level during the conflicting informants test trials (Maccurate = 6.69/12, S.E. = 0.48, t(15) = 1.43, p = 0.17, d = 0.36). There was also no evidence of learning across the conflicting informant test trials, with 11/16 dogs following the Accurate pointer to find the hidden treat on the first trial and 9/16 dogs following the Accurate pointer to find the hidden treat on trial 12, Β = − 0.015, S.E. = 0.01, t = − 1.46, p = 0.16. Dogs’ inability to distinguish between a previously accurate and a previously inaccurate informant when they are compared directly may suggest that dogs are not able to use humans’ previous pointing accuracy as a cue to their current accuracy, or to compare the relative past accuracy of two informants. However, this conclusion may be premature as there are some possible non-social alternative explanations for dogs’ inability to succeed in the conflicting informant test trials.
Dogs’ inability to succeed during the conflicting informants test trials in Experiment 2 could be attributable to confusion surrounding occlusion events, understanding the search task when they have not seen the food hidden, or needing to track two people simultaneously, all factors unrelated to the ability under investigation (for a similar argument, see Bensky et al. 2013).
Experiment 3
Experiment 3 aimed to reduce or rule out alternative causes of dogs’ failure to discriminate the two informants in the test trials of Experiment 2. We hypothesized that by first familiarizing dogs to the potentially challenging or confusing non-accuracy related components of the conflicting informants test trials (such as occlusion events, and the simultaneous presence of multiple informants), this might reveal improved ability to choose between informants on the basis of their prior accuracy. Further, if dogs once again did not demonstrate selective trust during the conflicting informants test trials, we could be more confident that dogs are failing due to an inability to use past informant accuracy as a choice metric.
Methods
Participants
The participants were 27 pet dogs of which 22 were included in analysis (male = 14; mean age = 52.01 months; range = 13.5–142.3 months). Five dogs were excluded from the study based on criteria determined prior to the start of data collection, (1) did not finish the first visit due to anxiety or lack of interest (n = 4), or (2) did not finish the second visit due to anxiety or lack of interest (n = 1).
An additional 5 dogs (for a total of 32) only participated in a first visit and were not able to be scheduled in for a second visit prior to the completion of data collection. All participants were recruited on a volunteer basis from the Greater Toronto Area and there were no breed requirements. Experiment 2 suggested that if dogs really can succeed at our task, the effect size may be smaller than 70% success. Therefore, rather than having an a priori fixed sample size, in Experiment 3 we used a statistically valid sequential analysis procedure with predetermined stopping criteria described in Frick (1998) and pre-registered this procedure on OSF prior to data collection.Footnote 5
In addition, following our pre-registered exclusion criteria, three (out of the final 22) dogs were excluded from the initial analysis to determine sample size, due to their poor performance on the non-social hidden baiting task in visit 2 (detailed below). This was due to our concern that dogs who were not able to succeed at finding a presently visible treat when the baiting was done out of their view might have broader difficulty with tasks involving occlusion or hidden baiting events and that this could confound the results of our final social task. See supplementary materials for a detailed breakdown of the excluded dogs.
Materials and procedure
The 2-piece occluder was identical to the one used in Experiment 2. Experiment 3 differed in using clear dishes (rather than cups) to cover the location of the treat for all but the conflicting informants' test trials, where identical opaque dishes were used. As the dishes were clear and were rubbed with treats to control for olfactory cues. All trials involved dogs locating a treat that was placed on a white plastic plate, lined to control for auditory cues and rubbed down with treats to control for olfactory cues. Experiment 3 took place in two separate visits comprising of four phases each (see Fig. 5). Visit 1 had warm-up, object permanence, occlusion-visible baiting, and occlusion-hidden baiting. Visit 2 had warm-up, occlusion-hidden baiting, informant history, and conflicting informants' test trials. As in Experiment 2, owners handled their dogs and received identical training. As in Experiment 2, informants wore either a black or white T-shirt (order counterbalanced) to help dogs distinguish them. Warm-up, informant history and test trials used the same procedure as their respective phases of Experiment 2, with the only change being that clear dishes were used in the warm-up and history trials (rather than opaque cups) and opaque dishes (rather than cups) were used in the test trials.
Procedures for the phases of Experiment 3. In Visit 1 dogs completed, in order, a warm-up, b object permanence, c occlusion-visible baiting, and d occlusion-hidden baiting. In visit 2, dogs completed, in order, a warm-up, d occlusion-hidden baiting, e informant history, and f conflicting informants' test trials
Treats were also placed on lined white plates rather than on the ground as in Experiment 1 and 2. All phases but the warm-up phase were completed over 12 trials and the location of the treat was pseudo-randomized between the left and right plates across trials, with the constraint that the treat appeared equally in both locations and did not appear on the same plate more than twice in a row.
The warm-up and object permanence trials used the same experimental procedure as Experiment 1. The occlusion trials were used to introduce the dog to the movement and placement of the occluder and to familiarize them with tracking the location of the treat across occlusion events. Occlusion-visible baiting used an identical procedure to the object permanence trials except that after both locations were covered the Experimenter dragged one piece of the two-piece occluder in front of the dog, and then dragged it back to its resting location on the side (Fig. 5).
The Experimenter verbally released the dog and released any tension in the leash after the occluder was out of the way and the Coder recorded which location the dog searched. Occlusion-hidden baiting familiarized dogs with baiting events that occurred out of their view behind the occluder. The Experimenter dragged the occluder into place, placed the treat on the plate (the dog’s view of the search locations was blocked by the occluder), covered both plates with the clear dishes, and then moved the occluder away, so that the treat was now visible under one of the dishes (Fig. 5). Occlusion-hidden baiting required dogs to locate the treat following the occluded baiting, by visually searching both locations, as they did not observe the baiting process. This process ensured that the dogs were familiar with searching for and locating treats even when they did not directly see the treats being placed.
Visit 2 began with the warm-up phase, with the only change from visit 1 being that the warm-up trials were conducted with both the clear dish as before (minimum 2 trials) and the occluded dish (minimum 2 trials). Occlusion-hidden baiting was then repeated using an identical procedure to visit 1 to confirm that dogs remembered the procedure across sessions. As discussed under participants, dogs who were not numerically above chance (> 6 out of 12 trials) during this phase were excluded from the initial confirmatory analysis for sample size.
Next, during the informant history trials and conflicting informants' test trials, informants were seated 0.75 m apart facing the dog at the start line (see Figs. 3 and 5e, f). The informant history trials introduced dogs to both of the Informants at the same time and to familiarize them with simultaneously tracking both informants. A trial began with informants moving their piece of the occluder into position blocking the dog’s view. The accurate informant then placed a treat under their clear dish and the inaccurate informant identically mirrored the movements but did not place a treat. The informants then removed the occluders revealing the two plates covered with clear dishes to the dog. The informants then both executed a static point at their respective dishes while simultaneously gazing at the dish and maintained this position until the dog made a choice. As in Experiment 2, the accurate informant always sat on the side of the treat to avoid informants pointing over one another and asymmetrical points. The conflicting informants’ test trials were identical to the informant history trials in the actions performed by the experimenters, but opaque dishes were used rather than clear. This change in dish opacity meant that dogs had no visual information of their own about the location of the treat and were forced to use their past knowledge about the accuracy of the informants from the history trials to make their decision.
Data scoring and analysis
Choice and accuracy criteria were the same as in Experiment 1 and 2. An additional coder who was blind to the purpose of the study’s hypothesis also coded for choice in 25% of videos to ensure that the data was recorded accurately. Recoded reliability was very high at 99.44%. As in Experiment 1 and 2, trials in which the subject did not make a choice within 30 s or where the experimenter made an error were repeated and the original trial was excluded from analysis. As in Experiment 1 and 2, Statistical analyses were conducted using R statistical software version 3.5.2 (R Development Core Team 2013). An initial confirmatory t test (to determine whether dogs are performing different than chance in the conflicting informants' test trials), and a mixed-effects logistic regression (examining trial number, history phase performance, and occlusion-hidden baiting performance as predictors of test trial outcome) were pre-registered to be conducted initially with only those dogs who scored above chance on the occlusion-hidden baiting task of visit 2. Subsequent versions of those analyses with all included dogs were also pre-registered. Finally, t tests comparing each of the phases against chance was pre-registered. In addition to these analyses, we also include analysis of dogs’ performance on the familiarization phases prior to the test trials. Analyses examining performance on tasks that occurred during visit 1 include all 28 dogs who completed all portions of visit 1. Analyses of tasks on visit 2 were first conducted with the 19 initially included dogs (as pre-registered), and then with all 22 dogs who completed all phases of visit 2. Including the 3 dogs who did not pass the initial inclusion criteria (passing the hidden baiting task) did not change the significance of any results. As a result, with the exception of the initial confirmatory analysis to determine final sample size, all analyses are reported with the full sample of 22 dogs.
Results and discussion
Asocial tasks
For all phases of visit 1, dogs performed significantly above chance, t ≥ 8.73, p < 0.001. (Fig. 6). Detailed analyses from visit 1 can be found in the supplementary materials. Dogs’ success in visit 1 is not surprising, as past studies have shown that dogs are able to flexibly go to search locations to locate visible treats in the absence of social cues (Kundey et al. 2010). In visit 2, dogs were still significantly above chance on the occlusion-hidden baiting task, (M = 8.82/12, S.E. = 0.51), t(21) = 5.50, p < 0.001, with 16/22 dogs locating the treat on the first trial and 17/22 dogs locating it on the last trial (Fig. 6).
Further, there was no significant difference between dogs’ performance on the hidden baiting task in visit 1 and visit 2, paired t-test, t(21) = 1.01, p = 0.32. This suggests that familiarization steps can be completed significantly earlier than test trials while still having their intended effect. The time between dogs’ first and second visits ranged from 8 to 196 days (M = 30.64, S.E. = 8.6). A correlation revealed that there was no impact on time between the scaffolding visit and test visit on test trial performance, r(20) = − 0.18, p = 0.42.
Social tasks
Consistent with their performance in Experiments 1 and 2, dogs were significantly above chance in the history phase (M = 9.41, S.E. = 0.43), t(21) = 7.84, p < 0.001. 19/22 dogs followed the accurate informant’s point and found the visible treat on the first trial and 16/22 dogs found the visible treat on the last trial.
Following our pre-registered initial confirmatory analysis, a one-sample t-test revealed that dogs were significantly above chance during the critical test trials when choosing the item indicated by the previously accurate versus the previously inaccurate informant, (M = 7, S.E. = 0.34), t(18) = 2.92, p = 0.009, d = 0.67. As discussed earlier, this initial analysis only took place with dogs (n = 19) who performed above numeric chance during the visit 2 occlusion-hidden baiting task. These results support the hypothesis that dogs are able to use past informant accuracy to locate hidden treats.
A follow-up t-test including the 3 dogs who were numerically at or below chance for occlusion-hidden baiting (n = 22) revealed that the results were not impacted, and dogs still performed significantly above chance on the conflicting informants' test trials (M = 6.95, S.E. = 0.3), t(21) = 3.21, p = 0.004, d = 0.68. As described in the Data Scoring and Analysis section, the remainder of analyses are reported with the full sample of 22 dogs. In addition, individually, 14 dogs performed numerically above chance across conflicting informants' test trials, while only 2 dogs were numerically below chance. Taken together we find that dogs are able to use informant past accuracy as a choice metric, but this is a difficult task for them.
In order to look at whether dogs were learning which informant to follow over the course of the session, we used a mixed-effects logistic regression model to evaluate performance across phases as a function trial number. Dogs performed consistently across trials and did not show evidence of learning across trials during any of the phases of visit 2, Β = 0.003, S.E. = 0.04, z = 0.07, p = 0.95. This supports the idea that dogs are not gradually learning to avoid one informant over trials but are using the informants’ previous accuracy to make their choice (Fig. 7).
In order to evaluate whether dogs who performed better on the familiarization and history tasks of visit 2 may have evaluated the informants better, a mixed-effects logistic regression predicting performance per trial in the test phase as a function of total successes during the history and occlusion-hidden baiting phases was conducted. This revealed that neither history phase performance, Β = 0.092, S.E. = 0.062, z = 1.46, p = 0.14, nor occlusion- hidden baiting performance, Β = -0.026, S.E. = 0.053, z = − 0.49, p = 0.62, predicted performance on the conflicting informants' test trials. These results suggest that poorer performance on the history phase does not necessarily reflect a poorer ability to track informant accuracy, and that those dogs who were performing better at the history phase were not necessarily learning more about the informants. Further, the results suggest that future studies could be less conservative on initial inclusion criteria, because poorer performance on the non-social tasks did not necessarily predict poorer performance on the social ones.
General discussion
Over three experiments we aimed to investigate whether domestic dogs could choose between human informants on the basis of their prior accuracy. In Experiment 1 dogs had to locate a hidden treat based on information from either an accurate or an inaccurate informant. Results are consistent with previous studies showing that dogs behave differently when faced with either an accurate versus an inaccurate informant (Petter et al. 2009; Kundey et al. 2010; Takaoka et al. 2015). Specifically, when dogs first saw a treat being hidden under one of two opaque cups, they were less likely to follow the point of an inaccurate informant who pointed away from the treat than an accurate informant who pointed toward the treat. These results provide support for the argument that dogs interpret pointing as a communicative cue, rather than as a command.
In Experiment 2, dogs first interacted with an accurate and an inaccurate informant separately. Consistent with Experiment 1, dogs behaved differently when presented individually with an accurate versus an inaccurate pointer and followed only the accurate pointer at above chance levels. Though dogs did not consistently ignore the inaccurate pointer, following their points at chance levels, these results further support the idea that dogs do not interpret pointing as an obligatory command. Following this, in the conflicting informant trials, dogs then had to choose which of the two informants’ conflicting points to follow when the location of the treat was unknown. Faced with this scenario, dogs chose randomly between searching the locations indicated by the previously accurate informant and the previously inaccurate informant.
In Experiment 3, the cognitive load of the task was reduced. Unlike in Experiment 2, after completing a series of tasks to familiarize them with the non-social components of the occlusion, baiting and choice procedures, dogs were able to follow the previously accurate pointer more often than the previously inaccurate pointer when presented with simultaneous conflicting information from both informants. This suggests that dogs are able to use past informant accuracy as a basis of choosing between human informants, and that their failure to do so in Experiment 2 was likely due to the additional cognitive demands of the test trials relative to the history trials, rather than an inability to distinguish between informants.
Together, these studies expand beyond past research to focus on a metric of social evaluation, namely selective trust on the basis of past accuracy, not previously explored in dogs. By using past accuracy, these studies explore dogs’ ability to incorporate their prior experience with an informant into their current decision making. In addition, by presenting dogs with two simultaneous points, dogs were presented with the opportunity to compare their relative trust in the two informants. Dogs’ success in Experiment 3 suggests a possible shared ability to engage in selective trust in social partners between humans and dogs, and a shared ability to track relative accuracy in particular.
For social animals who rely on others, it is advantageous to be able to use informants’ previous accuracy as a cue for choosing whose information to use. It is well established that young children are able to do this using a variety of appropriate paradigms including labelling novel objects and finding hidden items (Poulin-Dubois and Brosseau-Liard 2016). Dogs make an excellent species for investigating the evolutionary origins of this ability, given their unique understanding of human social cues and their demonstrated ability to discriminate between human informants on the basis of other characteristics including familiarity and informant knowledge (e.g., Agnetta et al. 2000; Hare et al. 2002; Kaminski and Nitzschner 2013). Our results suggest that, perhaps like great apes, who have been shown to be sensitive to informant past reliability in looking time tasks (Schmid et al. 2017), dogs may share humans’ ability to decide whose information to follow on the basis of their previous accuracy.
Dogs’ ability to use past pointing cues when making evaluations about informant accuracy distinguishes them from young children, who often struggle to override inaccurate pointing as compared to other inaccurate social cues such as verbal testimony (Palmquist and Jaswal 2012; Palmquist et al. 2018). Young children assume that pointers are knowledgeable (Palmquist and Jaswal 2012) and four-year-olds were unable to ignore a deceptive point, even when being told by a reliable speaker about the intentions of the deceiver (Palmquist et al. 2018). These results suggest that, for young children, pointing may be a particularly powerful cue, disrupting their evaluation of the informant’s information relative to their own. While dogs demonstrated an ability to use informant past accuracy in our task, they did not find our task easy. While clearly within the species capabilities of dogs, the significant asocial familiarization required in Experiment 3 alongside dogs’ failure in Experiment 2 means that we must be cautious in making broad generalizations about dogs’ ability to make choices based on informant past accuracy. Future work should investigate this social ability, and the extent to which it is readily available to dogs in different contexts.
In particular, inaccurate pointing may also be a particularly challenging cue for children to ignore as it is often regarded or presented experimentally as a deceptive cue. In our studies, and in much of the pointing literature, the inaccurate pointing informant is not mistaken. They demonstrate knowledge of where the item of interest is (by either looking into both containers or placing the item themselves), and in the case of the developmental work are often described to the child as actively trying to deceive them. This added component of reasoning about deceptive intentions of an unhelpful informant is known to be a much harder theory of mind task than the knowledge-based tasks that both dogs and children succeed at (Shafto et al. 2012). Intriguingly, there is some suggestive evidence that dogs may succeed at reasoning about deception, which may aid them in our task (Heberlein et al. 2017). Nonetheless, dogs were at a disadvantage in our task when compared to children as, unlike in the work in children, we were unable to give the dogs verbal information about the accuracy of the informants in advance or provide context about what is expected of them by verbally describing the choice task. Children are able to be scaffolded to a certain degree thanks to testimony standardly given by the researchers about what the task entails for the children (e.g., that they will first hear from two informants and should then select a location to get a sticker) as well as information about the informants, (such as telling the child that one informant is deceptive) (Palmquist et al. 2018). Dogs in our task must figure out the principles of the task, and form impressions of the informants without explicit instruction. Next steps might explore if there is a way to test dogs with a cue other than pointing, or to adapt a verbal paradigm for dogs, as pointing appears to be a particularly strong cue to children who find it particularly hard (relative to verbal testimony) to override with cues to inaccuracy (Palmquist and Jaswal 2012; Palmquist et al. 2018) and the same may be true for dogs.
Future work could also explore methods to provide dogs with more background information about the individual informants (e.g., observing third-party social interactions prior to trials) to better understand how dogs are evaluating conflicting social cues in their daily lives. Beyond presenting dogs with more information during the test session, future work could also explore the impact of preferences dogs may have (e.g., gender, age or other perceptual informant characteristics), that override or interact with prior accuracy. In an effort to control for possible confounding variables, our procedure intentionally made the informants as similar as possible, to avoid any possible preference that would influence the dogs’ responses, but future work could explore the potential impact of these preferences to make more generalizable conclusions. Taken together, dogs may perform differently if they are tested in a more natural environment that provides more information about individuals potentially related to their accuracy. Further, many of the asocial familiarization tasks required by our task would not be required in this more naturalistic context as they would presumably be completing a task they were already familiar with. Future studies could also test dogs across contexts to explore if there are certain situations where dogs rely more heavily on their own information as compared to that of a social informant (e.g., in an unfamiliar versus a familiar environment).
It is also not presently clear whether dogs view inaccurate pointers as deceptive or merely mistaken, but it is clear that the task of consistently choosing the accurate informant is difficult. If dogs are able to ignore deceptive informants, there are interesting and important implications for the study of theory of mind in dogs (Shafto et al. 2012). Researchers could also consider examining dogs’ understanding and evaluation of probabilistic social evidence. Our study utilized informants displaying deterministic cues (the informants were either 100% or 0% correct), and future work could borrow from work with children and present dogs with informants who are accurate 75% vs. 25% of the time to better explore how robust dogs’ abilities to discriminate on the basis of past accuracy are (Pasquini et al. 2007).
One potential concern could be that dogs are not discriminating the informants based on their past accuracy, but instead learn to discriminate which person or shirtcolour indicates food through associative learning.Footnote 6 While dogs are certainly capable of associative learning, we believe that this interpretation is unlikely for the following reasons. First, dogs generally take many trials (e.g., at least 27 in the case of Piotti et al. 2018), often over multiple sessions (multiple sessions of 30 trials each as seen in Wallis et al. 2016) to learn to discriminate between stimuli that predict the location of a food reward. In addition, dogs are not very adept at using visual markers to locate food in the absence of any human gestures (Agnetta et al. 2000; Udell et al. 2008). As such, if dogs were ignoring the informants’ pointing and only attending to the shirt color as a stimulus, it would likely take longer than 12 trials for them to succeed as they are in Experiment 3. Second, if dogs were learning an association between one person or color and the reward, then we would expect to see evidence of learning across the test trials or the history trials, something not observed in our experiments. We might also expect that dogs who were more successful in the history phase, and therefore had their choice reinforced more often, would form a stronger association, and be more successful in the test phase, but this relationship was also not observed. Taken together our results support the hypothesis that dogs’ are tracking and remembering the accuracy of the human pointers.
In conclusion, our findings add to the discourse on the ways in which dogs evaluate social information. Dogs, like young children, appear sensitive to the quality of information being provided about a hidden reward (Poulin-Dubois and Brosseau-Liard 2016; Schmid et al. 2017). Our work demonstrates that dogs are capable of integrating previous experience with accurate and inaccurate people into their decision making to make advantageous choices in the absence of their own personal information.
Code Availability
The code used to analyze the data are available on the Open Science Framework at: https://osf.io/dy83g/?view_only=d15980cb00bf4f91ab43edee2344708d
Notes
Original sample size was planned to be 16 per condition (see footnote 2). Sample size was chosen to provide power ≥ 80% for detecting a medium to large effect (average correct performance on 70% or more trials, Rosner, 2015). Calculations were completed via https://www.stat.ubc.ca/~rollin/stats/ssize/n1.html. Variance estimates were taken from internal data on previous tasks.
Original analyses were conducted using a different definition of side bias (10/12 to the same side) resulting in excluding 3 dogs from the Inaccurate condition for an even sample size of 16 in each condition and 32 total. Subsequent experiments used the definition presented here (12/12 to the same side) as we anticipated that they would be more difficult. Based on reviewer comments and in order to maintain consistency across experiments, we present the results here on the basis of the 12/12 side bias criteria. These results do not differ from the original results which are presented in the supplementary materials.
One dog saw the treat on the same side on three consecutive trials due to experimenter error, but this did not appear to impact performance (dog scored 11/12 with the missed trial occurring prior to the experimenter error).
The higher exclusion rate of this Experiment, relative to others, is partially due to 4 dogs who were excluded for reasons not pertaining to the experiment (specifically owner handling error and dog overheating due to room temperatures exceeding 80 Fahrenheit). Possible reasons for elevated exclusion rate of the remaining 8 dogs, namely the difficulty of the task and increased session length, will be returned to in the discussion and is further discussed in the supplementary materials.
Link to pre-registration: https://osf.io/vaq8w. In brief, in the procedure described by Frick (1998), an initial pre-determined sample size is collected, after which data collection continues until either p < 0.01 or p > 0.36 based on a pre-selected primary analysis. As discussed in our pre-registration, our initial sample size was 16 dogs, and the final sample size was determined using our primary analysis (a t test of the test trial performance of non-excluded dogs against chance). Our other secondary analyses were not examined until the full sample was collected. This procedure is approximately 30% more efficient in the number of subjects required when compared to a traditional fixed sample size, while not inflating Type I error rates. See our OSF pre-registration for additional details of our procedure, and Frick 1998 for additional statistical details.
Due to the COVID-19 global pandemic, we are unable to run a final control experiment examining dogs’ choices in the presence of informants who wear different shirt colors and do not point. Nonetheless, we feel confident in the results presented here, for the reasons presented in the main text.
References
Agnetta B, Hare B, Tomasello M (2000) Cues to food location that domestic dogs (Canis familiaris) of different ages do and do not use. AnimCogn 3(2):107–112. https://doi.org/10.1007/s100710000070
Bensky MK, Gosling SD, Sinn DL (2013) Chapter five—the world from a dog’s point of view: a review and synthesis of dog cognition research. In: Brockmann H, Roper T, Naguib M, Mitani J, Simmons L, Barrett L (eds) Advances in the study of behavior (Vol. 45 pp. 209–406). Academic Press, Cambridge
Bräuer J, Kaminski J, Riedel J, Call J, Tomasello M (2006) Making inferences about the location of hidden food: social dog, causal ape. J Comp Psychol 120(1):38–47. https://doi.org/10.1037/0735-7036.120.1.38
Catala A, Mang B, Wallis L, Huber L (2017) Dogs demonstrate perspective taking based on geometrical gaze following in a Guesser-Knower task. AnimCogn 20(4):581–589
Cook A, Arter J, Jacobs LF (2014) My owner, right or wrong: the effect of familiarity on the domestic dog’s behavior in a food-choice task. AnimCogn 17(2):461–470. https://doi.org/10.1007/s10071-013-0677-0
Corriveau KH, Meints K, Harris PL (2009) Early tracking of informant accuracy and inaccuracy. Br J Dev Psychol 27:331–342. https://doi.org/10.1348/026151008X310229
Dwyer C, Cole MR (2018) Domesticated dogs (Canis familiaris) tend to follow repeated deceptive human cues even when food is visible. Learn Behav 46(4):442–448. https://doi.org/10.3758/s13420-018-0356-8
Engelmann JM, Herrmann E (2016) Chimpanzees trust their friends. CurrBiol 26(2):252–256. https://doi.org/10.1016/j.cub.2015.11.037
Engelmann JM, Herrmann E, Tomasello M (2015) Chimpanzees trust conspecifics to engage in low-cost reciprocity. Proc R Soc B BiolSci 282:2014803. https://doi.org/10.1098/rspb.2014.2803
Frick RW (1998) A better stopping rule for conventional statistical tests. Behav Res Methods InstrumComput 30(4):690–697
Ganea PA, Koenig MA, Millett KG (2011) Changing your mind about things unseen: toddlers’ sensitivity to prior reliability. J Exp Child Psychol 109(4):445–453
Hare B, Tomasello M (1999) Domestic dogs (Canis familiaris) use human and conspecific social cues to locate hidden food. J Comp Psychol 113(2):173–177. https://doi.org/10.1037/0735-7036.113.2.173
Hare B, Brown M, Williamson C, Tomasello M (2002) The Domestication of social cognition in dogs. Science 298(5598):1634–1636. https://doi.org/10.1126/science.1072702
Harris PL, Koenig MA, Corriveau KH, Jaswal VK (2018) Cognitive foundations of learning from testimony. Annu Rev Psychol 69:251–273. https://doi.org/10.1146/annurev-psych-122216-011710
Heberlein MTE, Manser MB, Turner DC (2017) Deceptive-like behavior in dogs (Canis familiaris). AnimCogn 20(3):511–520. https://doi.org/10.1007/s10071-017-1078-6
Kaminski J, Nitzschner M (2013) Do dogs get the point? A review of dog–human communication ability. Learn Motiv 44(4):294–302
Kaminski J, Schulz L, Tomasello M (2012) How dogs know when communication is intended for them. Dev Sci 15(2):222–232. https://doi.org/10.1111/j.1467-7687.2011.01120.x
Kundey SMA, De Los Reyes A, Arbuthnot J, Allen R, Coshun A, Molina S, Royer E (2010) Domesticated dogs’ (Canis familiaris) response to dishonest human points. Int J Comp Psychol 23:201–215
Kundey SMA, De Los Reyes A, Royer E, Molina S, Monnier B, German R, Coshun A (2011) Reputation-like inference in domestic dogs (Canis familiaris). AnimCogn 14(2):291–302. https://doi.org/10.1007/s10071-010-0362-5
Lakatos G, Gácsi M, Topál J, Miklósi Á (2012) Comprehension and utilisation of pointing gestures and gazing in dog–human communication in relatively complex situations. AnimCogn 15(2):201–213. https://doi.org/10.1007/s10071-011-0446-x
Laland KN (2004) Social learning strategies. Anim Learn Behav 31(1):4–14. https://doi.org/10.3758/BF03196002
Lea SEG, Osthaus B (2018) In what sense are dogs special? Canine cognition in comparative context. Learn Behav 46:335–363. https://doi.org/10.3758/s13420-018-0349-7
Ma L, Ganea PA (2010) Dealing with conflicting information: young children’s reliance on what they see versus what they are told. Dev Sci 13(1):151–160. https://doi.org/10.1111/j.1467-7687.2009.00878.x
Maginnity ME, Grace RC (2014) Visual perspective taking by dogs (Canis familiaris) in a Guesser-Knower task: evidence for a canine theory of mind? AnimCogn 17(6):1375–1392
McKinley J, Sambrook TD (2000) Use of human-given cues by domestic dogs (Canis familiaris) and horses (Equus caballus). AnimCogn 3(1):13–22. https://doi.org/10.1007/s100710050046
Miklósi Á, Soproni K (2006) A comparative analysis of animals’ understanding of the human pointing gesture. AnimCogn 9(2):81–93. https://doi.org/10.1007/s10071-005-0008-1
Mills CM (2013) Knowing when to doubt: developing a critical stance when learning from others. Dev Psychol 49(3):404–418. https://doi.org/10.1037/a0029500
Nitzschner M, Kaminski J, Melis A, Tomasello M (2014) Side matters: potential mechanisms underlying dogs’ performance in a social eavesdropping paradigm. AnimBehav 90:263–271. https://doi.org/10.1016/j.anbehav.2014.01.035
Palmquist CM, Burns HE, Jaswal VK (2012) Pointing disrupts preschoolers’ ability to discriminate between knowledgeable and ignorant informants. Cogn Dev 27(1):54–63
Palmquist CM, Konrad RL, Norris MN (2018) Follow my point? Preschoolers’ expectations about veridicality disrupt their understanding of deceptive points. Cogn Dev 48:190–202
Pasquini ES, Corriveau KH, Koenig M, Harris PL (2007) Preschoolers monitor the relative accuracy of informants. Dev Psychol 43:1216–1226
Petter M, Musolino E, Roberts WA, Cole M (2009) Can dogs (Canis familiaris) detect human deception? BehavProc 82(2):109–118. https://doi.org/10.1016/j.beproc.2009.07.002
Piotti P, Szabó D, Bognár Z, Egerer A, Hulsbosch P, Carson RS, Kubinvi E (2018) Effect of age on discrimination learning, reversal learning, and cognitive bias in family dogs. Learn Behav 46:537–553. https://doi.org/10.3758/s13420-018-0357-7
Poulin-Dubois D, Brosseau-Liard P (2016) The developmental origins of selective social learning. Curr Direct PsycholSci 25(1):60–64. https://doi.org/10.1177/0963721415613962
Povinelli DJ, Nelson KE, Boysen ST (1990) Inferences about guessing and knowing by Chimpanzees (Pan troglodytes). J Comp Psychol 104(3):203–210
R Development Core Team (2013). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/.
Rosner B (2015) Fundamentals of Biostatistics. Cengage Learning
Scheider L, Grassmann S, Kaminski J, Tomasello M (2011) Domestic dogs use contextual information and tone of voice when following a human pointing gesture. PLoS One 6(7): e21676. https://doi.org/10.1371/Fjournal.pone.0021676
Scheider L, Kaminski J, Call J, Tomasello M (2013) Do domestic dogs interpret pointing as a command? AnimCogn 16(3):361–372. https://doi.org/10.1007/s10071-012-0577-8
Schmid B, Karg K, Perner J, Tomasello M (2017) Great apes are sensitive to prior reliability of an informant in a gaze following task. PLoS ONE 12(11):e0187451. https://doi.org/10.1371/journal.pone.0187451
Shafto P, Eaves B, Navarro DJ, Perfors A (2012) Epistemic trust: modeling children’s reasoning about others’ knowledge and intent. Dev Sci 15(3):436–447. https://doi.org/10.1111/j.1467-7687.2012.01135.x
Silver ZA, Furlong EE, Johnston AM, Santos LR (2020) Training differences predict dogs’ (Canis lupus familiaris) preferences for prosocial others. AnimCogn. https://doi.org/10.1007/s10071-020-01417-9
Szetei V, Miklósi Á, Topál J, Csányi V (2003) When dogs seem to lose their nose: an investigation on the use of visual and olfactory cues in communicative context between dog and owner. ApplAnimBehavSci 83(2):141–152. https://doi.org/10.1016/S0168-1591(03)00114-X
Takaoka A, Maeda T, Hori Y, Fujita K (2015) Do dogs follow behavioral cues from an unreliable human? AnimCogn 18(2):475–483. https://doi.org/10.1007/s10071-014-0816-2
Topál J, Gergely G, Erdőhegyi Á, Csibra G, Miklósi Á (2009) Differential sensitivity to human communication in dogs, wolves, and human infants. Science 325(5945):1269. https://doi.org/10.1126/science.1176960
Udell MAR, Giglio RF, Wynne CDL (2008) Domestic dogs (Canis familiaris) use human gestures but not nonhuman tokens to find hidden food. J Comp Psychol 122(1):84–93. https://doi.org/10.1037/0735-7036.122.1.84
Wallis LJ, Virányi Z, Müller CA, Serisier S, Huber L, Range F (2016) Aging effects on discrimination learning, logical reasoning and memory in pet dogs. Age 38(1):6. https://doi.org/10.1007/s11357-015-9866-x
Wynne CDL, Udell MAR, Lord KA (2008) Ontogeny's impacts on human-dog communication. Anim Behav 76:e1–e4. https://doi.org/10.1016/j.anbehav.2008.03.010
Acknowledgements
Thanks to University College at the University of Toronto for providing our testing space. Thanks to the members of the U of T Canine Cognition Lab for their assistance with data collection. We also thank the dogs and their humans for their participation in the project. This research was funded by the Natural Sciences and Engineering Research Council of Canada [Grant Number 05552, 2016].
Funding
This research was funded by the Natural Sciences and Engineering Research Council of Canada [Grant Number 05552, 2016].
Author information
Authors and Affiliations
Contributions
MHP: methodology, investigation, visualization, project administration, writing—original draft, reviewing & editing, formal analysis. JE: methodology, investigation, writing – review & editing, project administration, visualization, formal analysis. ECT: conceptualization, methodology, writing – review & editing. SMM: methodology, investigation. angie johnston: conceptualization, methodology. DB: supervision, writing – reviewing and editing, conceptualization, methodology, formal analysis.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflicts of interest.
Ethical standards
All three experiments were approved by the University of Toronto’s University Animal Care Committee (UACC). Procedures across all three experiments were in accordance with Ontario’s Animals for Research Act, the federal Canadian Council on Animal Care and fully complied with the APA Ethical Standards for Use of Animals in Research.
Availability of data and material
The data collected and analyzed are available on the Open Science Framework at: https://osf.io/dy83g/?view_only=d15980cb00bf4f91ab43edee2344708d
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pelgrim, M.H., Espinosa, J., Tecwyn, E.C. et al. What’s the point? Domestic dogs’ sensitivity to the accuracy of human informants. Anim Cogn 24, 281–297 (2021). https://doi.org/10.1007/s10071-021-01493-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-021-01493-5