Abstract
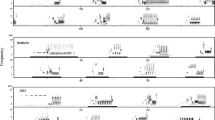
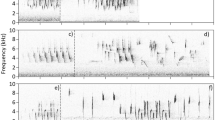
Songbirds have been traditionally classified into close-ended or open-ended learning species according to the length of the sensitive period during which birds are able to memorize new vocalizations. Closed-ended learners are generally not capable of changing their song after the first year of life, while open-ended learners show song plasticity as adults. A few Turdus species have been suggested to be open-ended learners, but no long-term study has been conducted to investigate their song plasticity over time. We analyzed the songs of clay-colored thrushes, T. grayi, over four successive breeding seasons to assess song plasticity in their syllable repertoires within and between breeding seasons. A total of 16,262 syllables were classified through visual inspection of spectrograms and multidimensional scaling analysis based on spectrogram correlations. On average, 563 ± 153 (SD) syllables per male per breeding season were analyzed. Male repertoire size was 9–20 syllable types. Males were capable of modifying their syllable repertoire between the initial and final periods of the breeding season. Song plasticity within breeding seasons may be associated with imitation between neighboring males, suggesting song learning in males that were ≥2 years old. This short-term plasticity is not enough, however, to explain the high proportion of change (mean = 65 % syllable types) in repertoire composition between breeding seasons in adult males. Song plasticity resulting from annual changes in repertoire composition could be explained by open-ended learning, but another mechanism, extended memory and re-expression, could also explain long-term plasticity. Experimental studies controlling the acoustic environment are needed to determine which mechanism is responsible for such a high level of song plasticity.








Similar content being viewed by others
References
Araya-Salas M, Wright T (2013) Open ended song learning in a hummingbird. Biol Lett 9:20130625
Baptista LF, Schuchmann KL (1990) Song learning in the Anna Hummingbird (Calypte anna). Ethology 84:15–26
Beecher MD, Burt J (2004) The role of social interaction in bird song learning. Curr Dir Psychol Sci 13:224–228
Brainard MS, Doupe AJ (2013) Translating birdsong: songbirds as a model for basic and applied medical research. Annu Rev Neurosci 36:489–517
Brenowitz E, Beecher MD (2005) Song learning in birds: diversity and plasticity, opportunities and challenges. Trends Neurosci 28:127–132
Buchanan KL, Catchpole CK (1997) Female choice in the sedge warbler, Acrocephalus schoenobaenus: multiple cues from song and territory quality. Proc R Soc B 264:521–526
Catchpole CK, Slater PJR (2008) Bird song biological themes and variations, 2nd edn. Cambridge University Press, Cambridge
Chaiken M, Bohner J, Marler P (1994) Repertoire turnover and the timing of song acquisition in European starlings. Behaviour 128:25–39
Charif RA, Waack AM, Strickman AM (2010) Raven Pro 1.4 user’s manual. Cornell Lab of Ornithology, Ithaca
Clement P (2001) Thrushes. Princeton University Press, Princeton
Cramer ERA (2013) Measuring consistency: spectrogram cross-correlation versus targeted acoustic parameters. Bioacoustics 22:247–257
Dabelsteen T, McGregor PK, Lampe HM, Langmore NE, Holland J (1998) Quiet song in song birds: an overlooked phenomenon. Bioacoustics 9:89–105
Devoogd TJ, Krebs JR, Healy SD, Purvis A (1993) Relations between song repertoire size and the volume of brain nuclei related to song: comparative evolutionary analyses amongst oscine birds. Proc R Soc B 254:75–82
DeWolfe BB, Baptista LF, Petrinovich L (1989) Song development and territory establishment in Nuttall’s white-crowned sparrows. Condor 91:397–407
Dyrcz A (1983) Breeding ecology of the clay-coloured Robin Turdus grayi in lowland Panama. Ibis 125:287–304
Eriksen A, Slagsvold T, Lampe HM (2011) Vocal plasticity—are pied flycatchers, Ficedula hypoleuca, open-ended learners? Ethology 117:188–198
Espmark YO, Lampe HM (1993) Variations in the song of the pied flycatcher within and between breeding seasons. Bioacoustics 5:33–65
Farabaugh SM, Dooling RJ (1996) Acoustic communication in parrots: laboratory and field studies of budgerigars, Melopsittacus undulates. In: Kroodsma DE, Miller EH (eds) Ecology and evolution of acoustic communication in birds. Comstock Publishing Associates, New York, pp 97–117
Franco P, Slabbekoorn H (2009) Repertoire size and composition in great tits: a flexibility test using playbacks. Anim Behav 77:261–269
Gaunt SLL, Baptista LF, Sánchez JE, Hernandez D (1994) Song learning as evidenced from song sharing in two hummingbird species (Colibri coruscans and C. thalassinus). Auk 111:87–103
Geberzahn N, Hultsch H, Todt D (2002) Latent song type memories are accessible through auditory stimulation in a hand-reared songbird. Anim Behav 64:783–790
Gil D, Gahr M (2002) The honesty of bird song: multiple constraints for multiple traits. Trends Ecol Evol 17:133–141
Gil D, Slater PJB (2000) Song organisation and singing patterns of the willow warbler, Phylloscopus trochilus. Behaviour 137:759–782
Gil D, Cobb JLS, Slater PJB (2001) Song characteristics are age dependent in the willow warbler, Phylloscopus trochilus. Anim Behav 62:689–694
Grabowski GL (1979) Vocalizations of the Rufous-backed thrush (Turdus rufopalliatus) in Guerrero, Mexico. Condor 81:409–416
Hough GE, Nelson DA, Volman SF (2000) Re-expression of songs deleted during vocal development in white-crowned sparrows, Zonotrichia leucophrys. Anim Behav 60:279–287
Howard RD (1974) The influence of sexual selection and interspecific competition on mockingbird song (Mimus polyglottos). Evolution 28:428–438
Johnson S (2006) Do American Robins acquire songs by both imitating and inventing? Wilson J Ornithol 118:341–352
Kiefer S, Sommer C, Scharff C, Kipper S, Mundry R (2009) Tuning towards tomorrow? Common nightingales Luscinia megarhynchos change and increase their song repertoires from the first to the second breeding season. J Avian Biol 40:231–236
Kipper S, Kiefer S (2010) Age-related changes in birds’ singing styles: on fresh tunes and fading voices? Adv Stud Behav 41:77–118
Kipper S, Mundry R, Hultsch H, Todt D (2004) Long-term persistence of song performance rules in nightingales (Luscinia megarhynchos): a longitudinal field study on repertoire size and composition. Behaviour 141:371–390
Krebs J, Ashcroft R, Webber M (1978) Song repertoires and territory defence in the great tit. Nature 271:539–542
Kroodsma DE (1982) Learning and the ontogeny of sound signals in birds. In: Kroodsma DE, Miller EH (eds) Acoustic communication in birds, vol 2, song learning and its consequences. Academic Press, Boston, pp 1–24
Kroodsma DE, Liu W, Goodwin E, Bedell P (1999) The ecology of song improvisation as illustrated by North American Sedge Wrens. Auk 116:373–386
Lampe HM, Saetre G (1995) Female pied flycatchers prefer males with larger song repertoires. Proc R Soc B 262:163–167
Leitner S, Voigt C, Gahr M (2001) Seasonal changes in the song pattern of the non-domesticated island canary (Serinus canaria), a field study. Behaviour 138:885–904
Lynch A (1996) The population memetics of birdsong. In: Kroodsma DE, Miller EH (eds) Ecology and evolution of acoustic communication in birds. Comstock Publishing Associates, New York, pp 181–197
Marler P (1970) A comparative approach to vocal learning: song development in white-crowned sparrows. Journal of Comparative and Physiological Psychology Monograph 71:1–25
Marler P, Peters S (1981) Sparrows learn adult song and more from memory. Science 213:780–782
Marler P, Peters S (1982) Subsong and plastic song: their role in the vocal learning process. In: Kroodsma DE, Miller EH (eds) Acoustic communication in birds, vol 2, song learning and its consequences. Academic Press, Boston, pp 25–50
Marler P, Mundinger P, Waser MS, Lutjen A (1972) Effects of acoustical stimulation and deprivation on song development in red-winged blackbirds (Agelaius phoeniceus). Anim Behav 20:586–606
McGregor PK, Krebs JR (1982) Mating and song types in the great tit. Nature 297:60–61
Molles LE, Vehrencamp SL (1999) Repertoire size, repertoire overlap, and singing modes in the Banded Wren (Thryothorus pleurostictus). Auk 116:677–689
Mountjoy DJ, Lemon RE (1995) Extended song learning in wild European starlings. Anim Behav 49:357–366
Nelson DA (1992) Song overproduction and selective attrition lead to song sharing in the field sparrow (Spizella pusilla). Behav Ecol Sociobiol 30:415–424
Nicholson JS, Buchanan KL, Marshall RC, Catchpole CK (2007) Song sharing and repertoire size in the sedge warbler, Acrocephalus schoenobaenus: changes within and between years. Anim Behav 74:1585–1592
Nordby JC, Campbell SE, Beecher MD (2001) Late song learning in song sparrows. Anim Behav 61:835–846
Nottebohm F (2004) The road we travelled: discovery, choreography, and significance of brain replaceable neurons. Ann NY Acad Sci 1016:628–658
Nottebohm F, Nottebohm ME, Crane L (1986) Development and seasonal changes in canary song and their relation to changes in the anatomy of song-control nuclei. Behav Neural Biol 46:445–471
Nowicki S, Nelson DA (1990) Defining natural categories in acoustic signals: comparison of three methods applied to ‘chick-a-dee’ call notes. Ethology 86:89–101
Payne RB (1996) Song traditions in indigo buntings: origin, improvisation, dispersal, and extinction in cultural evolution. In: Kroodsma DE, Miller EH (eds) Ecology and evolution of acoustic communication in birds. Comstock Publishing Associates, New York, pp 198–220
Payne RB, Payne LL (1997) Field observations, experimental design, and the time and place of learning bird songs. In: Snowdon CT, Hausberger M (eds) Social influences on vocal development. Cambridge University Press, Cambridge, pp 57–84
Pfaff JA, Zanette L, MacDougall-Shackleton SA, MacDougall-Shackleton EA (2007) Song repertoire size varies with HVC volume and is indicative of male quality in song sparrows (Melospiza melodia). Proc R Soc B 274:2035–2040
Rassmussen R, Dabelsteen T (2002) Song repertoires and repertoire sharing in a local group of blackbirds. Bioacoustics 13:63–76
R Development Core Team (2012) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. ISBN: 3-900051-07-0. http://www.R-project.org/
Saranathan V, Hamilton D, Powell GVN, Kroodsma DE, Prum RO (2007) Genetic evidence supports song learning in the three-wattled bellbird Procnias tricarunculata (Cotingidae). Mol Ecol 16:3689–3702
Searcy WA (1992) Song repertoire and mate choice in birds. Am Zool 32:71–80
Searcy WA, Andersson M (1986) Sexual selection and the evolution of song. Annu Rev Ecol Syst 17:507–533
Searcy WA, Beecher MD (2009) Song as an aggressive signal in songbirds. Anim Behav 78:1282–1292
Sorjonen J (1987) Temporal and spatial differences in traditions and repertoires in the song of the thrush nightingale (Luscinia luscinia). Behaviour 102:196–212
Stiles FG, Skutch AF (1989) A guide to the birds of Costa Rica. Cornell University Press, Ithaca
Stutchbury BJM, Morton ES, Piper WH (1998) Extra-pair mating system of a synchronously breeding tropical songbird. J Avian Biol 29:72–78
Sung H, Park S (2005) Explaining avian vocalizations: a review of song learning and song communication in male-male interactions. Integrative Biosciences 9:47–55
Todt D, Geberzahn N (2003) Age-dependent effects of song exposure: song crystallization sets a boundary between fast and delayed vocal imitation. Anim Behav 65:971–979
Vargas-Castro LE (2015) Spatial pattern of syllable sharing in white-throated thrushes: implications for song learning and dispersal behaviours. Behaviour 152:775–795
Vargas-Castro LE, Sánchez NV, Barrantes G (2012) Repertoire size and syllable sharing in the song of the clay-colored Thrush (Turdus grayi). Wilson J Ornithol 124:446–453
Zar JH (1996) Biostatistical analysis, 3rd edn. Prentice Hall, New Jersey
Acknowledgments
We thank the Oficina de Seguridad y Transito of the University of Costa Rica for providing logistical support. Bill Eberhard, Eric Fuchs, and Bill Searcy made helpful comments on earlier versions of the manuscript. Laura Strickman and Dean Hawthorne helped to configure a sound detector in Raven. Jeff Woodman and Tim Burr provided recording equipment.
Ethical standard
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vargas-Castro, L.E., Sánchez, N.V. & Barrantes, G. Song plasticity over time and vocal learning in clay-colored thrushes. Anim Cogn 18, 1113–1123 (2015). https://doi.org/10.1007/s10071-015-0883-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-015-0883-z