Abstract
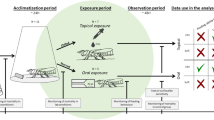
If animals are trained with two similar stimuli such that one is rewarding (S+) and one punishing (S−), then following training animals show a greatest preference not for the S+, but for a novel stimulus that is slightly more different from the S− than the S+ is. This peak shift phenomenon has been widely reported for vertebrates and has recently been demonstrated for bumblebees and honey bees. To explore the nature of peak shift in invertebrates further, here we examined the properties of peak shift in honey bees trained in a free-flight olfactory learning assay. Hexanal and heptanol were mixed in different ratios to create a continuum of odour stimuli. Bees were trained to artificial flowers such that one odour mixture was rewarded with 2 molar sucrose (S+), and one punished with distasteful quinine (S−). After training, bees were given a non-rewarded preference test with five different mixtures of hexanal and heptanol. Following training bees’ maximal preference was for an odour mixture slightly more distinct from the S− than the trained S+. This effect was not seen if bees were initially trained with two distinct odours, replicating the classic features of peak shift reported for vertebrates. We propose a conceptual model of how peak shift might occur in honey bees. We argue that peak shift does not require any higher level of processing than the known olfactory learning circuitry of the bee brain and suggest that peak shift is a very general feature of discrimination learning.




Similar content being viewed by others
References
Avarguès-Weber A, de Brito Sanchez MG, Giurfa M, Dyer AG (2010) Aversive reinforcement improves visual discrimination learning in free-flying honeybees. PLoS One 5:e15370. doi:10.1371/journal.pone.0015370
Bazhenov M, Huerta R, Smith B (2013) A computational framework for understanding decision making through integration of basic learning rules. J Neurosci 33:5686–5697
Bicker G, Schäfer S, Kingan T (1985) Mushroom body feedback interneurones in the honeybee show GABA-like immunoreactivity. Brain Res 360:394–397
Cassenaer S, Laurent G (2012) Conditional modulation of spike-timing-dependent plasticity for olfactory learning. Nature 482:47–52. doi:10.1038/nature10776
Cheng K (2002) Generalisation: mechanistic and functional explanations. Anim Cogn 5:33–40
Cheng K, Spetch ML (2002) Spatial generalization and peak shift in humans. Learn Motiv 33:358–389. doi:10.1016/s0023-9690(02)00003-6
Cheng K, Spetch ML, Johnston M (1997) Spatial peak shift and generalization in pigeons. J Exp Psychol Anim Behav Process 23:469–481
Daly K, Chandra S, Durtschi M, Smith BH (2001) The generalization of an olfactory-based conditioned response reveals unique but overlapping odour representations in the moth Manduca sexta. J Exp Biol 203:3085–3095
Deisig N, Lachnit H, Giurfa M (2002) The effect of similarity between elemental stimuli and compounds in olfactory patterning discriminations. Learn Mem 9:112–121
Dyer AG, Rosa MGP, Reser DH (2008) Honeybees can recognise images of complex natural scenes for use as potential landmarks. J Exp Biol 211:1180–1186
Gamberale G, Tullberg BS (1996) Evidence for a peak-shift in predator generalization among aposematic prey. Proc R Soc Lond B 263:1329–1334
Ghirlanda S, Enquist M (1998) Artificial neural networks as models of stimulus control. Anim Behav 56:1383–1389
Ghirlanda S, Enquist M (2003) A century of generalization. Anim Behav 66:15–36
Giurfa M, Sandoz JC (2012) Invertebrate learning and memory: fifty years of olfactory conditioning of the proboscis extension response in honeybees. Learn Mem 19:54–66. doi:10.1101/lm.024711.111
Gronenberg W (1987) Anatomical and physiological properties of feedback neurons of the mushroom bodies in the bee brain. J Exp Biol 46:115–125
Grünewald B (1999a) Morphology of feedback neurons in the mushroom body of the honeybee Apis mellifera. J Comp Neurol 404:114–126
Grünewald B (1999b) Physiological properties and response modulations of mushroom body feedback neurons during olfactory learning in the honeybee Apis mellifera. J Comp Physiol A 185:565–576
Grusec T (1968) The peak shift in stimulus generalization: equivalent effects of errors and noncontingent shock. J Exp Anal Behav 11:239–249
Guerrieri F, Schubert M, Sandoz J-C, Giurfa M (2005) Perceptual and neural olfactory similarity in honeybees. PLoS Biol 3:e60
Hanson HM (1959) Effects of discrimination training on generalization. J Exp Psychol 51:79–88
Hovland CI (1937) The generalisation of conditioned responses. I. The sensory generalisation of conditioned responses with varying frequencies of tone. J Gen Psychol 17:125–148
Huerta R (2012) Learning pattern recognition and decision making in the insect brain. Paper presented at the proceedings of the 12th Granada seminar on computational and statistical physics, La Herradura, Spain
Huerta R, Nowotny T (2009) Fast and robust learning by reinforcement signals: explorations in the insect brain. Neural Comput 24:2473–2507
Huerta R, Nowotny T, Garcia-Sanchez M, Abarbanel HDL, Rabinovich MI (2004) Learning classification in the olfactory system of insects. Neural Comput 16:1601–1640
Huerta R, Amigo JM, Nowotny T, Elkan C (2012) Inhibition in multiclass classification. Neural Comput 24:2473–2507
Ito I, Ong RCY, Raman B, Stopfer M (2008) Sparse odor representation and olfactory learning. Nat Neurosci 11:1177–1184. doi:10.1038/nn.2192
Laska M, Galizia C, Giurfa M, Menzel R (1999) Olfactory discrimination ability and odor structure–activity relationships in honeybees. Chem Senses 24:429–438
Leimar O, Tuomi J (1998) Synergistic selection and graded traits. Evol Ecol 12:59–71
Leonard AS, Dornhaus A, Papaj DR (2011) Flowers help bees cope with uncertainty: signal detection and the function of floral complexity. J Exp Biol 214:113–121. doi:10.1242/jeb.047407
Lynn SK, Cnaani J, Papaj DR (2005) Peak shift discrimination learning as a mechanism of signal evolution. Evolution 59:1300–1305. doi:10.1554/04-284
Mackintosh MJ (1974) The psychology of animal learning. Academic Press, London
McLaren IPL, Mackintosh NJ (2002) Associative learning and elemental representation: II. Generalization and discrimination. Anim Learn Behav 30:177–200. doi:10.3758/bf03192828
Menzel R (2001) Searching for the memory trace in a mini-brain, the honeybee. Learn Mem 8:53–62
Menzel R, Giurfa M (2001) Cognitive architecture of a mini-brain: the honeybee. Trends Cogn Sci 5:62–71
Menzel R, Müller U (1996) Learning and memory in honeybees: from behavior to neural substrates. Ann Rev Neurosci 19:379–404
Menzel R, Gumbert A, Kunze J, Shmida A, Vorobyev M (1997) Pollinators’ strategies in finding flowers. Isr J Plant Sci 45:141–156
Okada R, Rybak J, Manz G, Menzel R (2007) Learning-related plasticity in PE1 and other mushroom body-extrinsic neurons in the honeybee brain. J Neurosci 27:11736–11747. doi:10.1523/jneurosci.2216-07.2007
Paldi N, Zilber S, Shafir S (2003) Associative olfactory learning of honeybees to differential rewards in multiple contexts: effect of odor component and mixture similarity. J Chem Ecol 29:2515–2538
Pavlov IP (1927) Conditioned reflexes. Oxford University Press, Oxford
Perez-Orive J, Mazor O, Turner GC, Cassenaer S, Wilson RI, Laurent G (2002) Oscillations and sparsening of odor representations in the mushroom body. Science 297:359–365. doi:10.1126/science.1070502
Perry CJ, Barron AB (2013) Neural mechanisms of reward in insects. Ann Rev Entomol 58:543–562
Rybak J, Menzel R (1993) Anatomy of the mushroom bodies in the honey bee brain—the neuronal connections of the alpha-lobe. J Comp Neurol 334:444–465. doi:10.1002/cne.903340309
Spence KW (1937) The differential response in animals to stimuli varying within a single dimension. Psychol Rev 44:430–444
Strube-Bloss MF, Nawrot MP, Menzel R (2012) Mushroom body output neurons encode odor–reward associations. J Neurosci 31:3129–3140. doi:10.1523/jneurosci.2583-10.2011
Szyszka P, Galkin A, Menzel R (2008) Associative and non-associative plasticity in Kenyon cells of the honeybee mushroom body. Front Syst Neurosci 2. Article 3
Terrace HS (1968) Discrimination learning, the peak shift, and behavioral contrast. J Exp Anal Behav 11:727–741
Turner GC, Bazhenov M, Laurent G (2008) Olfactory representations by Drosophila mushroom body neurons. J Neurophysiol 99:734–746. doi:10.1152/jn.01283.2007
Weary D, Guilford T, Weisman R (1993) A product of discriminative learning may lead to female preferences for elaborate males. Evolution 47:333–336
Wright GA, Kottcamp S, Thompson MGA (2008) Generalization mediates sensitivity to complex odor features in the honeybee. PLoS One 3:e1704. doi:10.1371/journal.pone.0001704
Wright GA, Choudhary AF, Bentley MA (2009) Reward quality influences the development of learned olfactory biases in honeybees. Proc R Soc Lond B 276:2597–2604
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Andrew, S.C., Perry, C.J., Barron, A.B. et al. Peak shift in honey bee olfactory learning. Anim Cogn 17, 1177–1186 (2014). https://doi.org/10.1007/s10071-014-0750-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-014-0750-3