Abstract
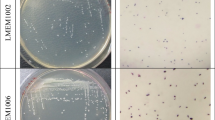
The objectives of this study were to deduce the effect of Piper betle leaves on lactofermentation of the idli batter, characterize the potent lactic acid bacteria (LAB) and elucidate their beneficial properties in soymilk fermentation. Twenty potent LAB isolates (11 bacilli and 9 cocci) showing potent antibacterial activity against food borne pathogens were obtained from the seventy isolates screened. The 20 isolates identified using 16S rRNA and Multiplex PCR, belonged to Lactobacillus plantarum subsp. argentoratensis, Lactobacillus paraplantarum, and Pediococcus pentosaceus. Among the 20 isolates, KJBC11, KJBB56, KJBB37, and KJBC67 exhibited high phytase activity in phytate hydrolysis. The isolates, KJBC67, KJBC06, and KJBB56 were used in soymilk fermentation which showed considerable reduction in the polyphenol content and trypsin inhibitory activity. The isolates KJBB37 and KJBC04 showed about 56% DPPH scavenging activity in LAB fermented soymilk compared to unfermented soymilk. Thus, this study provides the beneficial potentials of LAB isolates.
Similar content being viewed by others
References
Jamuna M, Jeevaratnam K. Isolation and characterization of lactobacilli from some traditional fermented foods and evaluation of the bacteriocins. J. Gen. Appl. Microbiol. 50: 79–90 (2004)
Jeevaratnam K, Jamuna M, Bawa A. Biological preservation of foods-Bacteriocins of lactic acid bacteria. Indian J. Biotechnol. 4: 446–454 (2005)
Chelule P, Mokoena M, Gqaleni N. Advantages of traditional lactic acid bacteria fermentation of food in Africa. Cur. Res. Technol. Educ. Topics Appl. Microbiol. Microb. Biotechnol. 1: 1160–1167 (2010)
Swain MR, Anandharaj M, Ray RC, Parveen Rani R. Fermented fruits and vegetables of Asia: A potential source of probiotics. Biotechnol. Res. Int. Article ID 250424 (2014)
Vidhyasagar V, Jeevaratnam K. Isolation and Characterization of Pediococcus pentosaceus from idly batter: A traditional South Indian fermented food source. Biosci. Biotechnol. Res. Asia 9: 427–431 (2012)
Agaliya PJ, Jeevaratnam K. Molecular characterization of lactobacilli isolated from fermented idli batter. Braz. J. Microbiol. 44: 1199–1206 (2013)
Bissa S, Songara D, Bohra A. Traditions in oral hygiene: Chewing of betel (Piper betle L.) leaves. Current Sci. 92: 6–28 (2007)
Pradhan D, Suri K, Pradhan D, Biswasroy P. Golden heart of nature: Piper betle leaf. J. Pharmacogn. Phytochem. 1: 147–167 (2013)
Greeshma AG, Srivastava B, Srivastava K. Plants used as antimicrobials in the preparation of traditional starter cultures of fermentation by certain tribes of Arunachal Pradesh. Bull. Arunachal Forest Res. 22: 52–57 (2006)
Michaylova M, Minkova S, Kimura K, Sasaki T, Isawa K. Isolation and characterization of Lactobacillus delbrueckii ssp. bulgaricus and Streptococcus thermophilus from plants in Bulgaria. FEMS Microbiol. Lett. 269: 160–169 (2007)
Park SY, Lee KD, An HM, Kim RJ, Kim MJ, Cha MK, Lee SW, Kim SO, Choi K, Lee K, Ha N. Producing functional soy yoghurt incubated with Bifidobacterium longum SPM1205 isolated from young adult Koreans. Biotechnol. Biotech. Eq. 26: 2759–2764 (2012)
Wang YC, Yu RC, Chou C. Antioxidative activities of soymilk fermented with lactic acid bacteria and bifidobacteria. Food Microbiol. 23: 128–135 (2006)
Yuan JP, Wang JH, Liu X. Metabolism of dietary soy isoflavones toequol by human intestinal micro flora-implications for health. Mol. Nutr. Food Res. 51: 765–781 (2007)
Zhang X, Qi JR, Li KK, Yin SW, Wang JM, Zhu JH, XQ Y. Characterization of soybeta-conglycinin-dextran conjugate prepared by Maillard reaction in crowded liquid system. Food Res. Int. 49: 648–654 (2012)
Liener IE. Possible adverse effects of soybean anticarcinogens. J. Nutr. 125: 744S–750S (1995)
Gupta YP. Anti-nutritional and toxic factors in food legumes: A review. Plant Food Hum. Nutr. 37: 201–228 (1987)
Vijayendra S, Rajashree K, Halami P. Characterization of a heat stable antilisterial bacteriocin produced by vancomycin sensitive Enterococcus faecium isolated from idli batter. Indian J. Microbiol. 50: 243–246 (2010)
Mundt JO. Bergey's Manual of Systematic Bacteriology. Vol. 2, pp. 1063–1071. In: Enterococci. Sneath PH, Mair NS, Sharpe EM, Holt GH (eds). Williams and Wilkins, Baltimore, MD, USA (1986)
Vijai Pal, Jamuna M, Jeevaratnam K. Isolation and characterization of bacteriocin producing lactic acid bacteria from a south Indian special dosa (Appam) batter. J. Culture Coll. 4: 53–60 (2005)
Pospiech A, Neumann B. A versatile quick-prep of genomic DNA from grampositive bacteria. Trends Genet. 11: 217–218 (1995)
Bonomo M, Ricciardi A, Zotta T, Parente E, Salzao G. Molecular and technological characterization of lactic acid bacteria from traditional fermented sausages of Brasilicata region (Southern Italy). Meat Sci. 80: 1238–1248 (2008)
Saraniya A, Jeevaratnam K. Molecular characterization of bacteriocinogenic Lactobacillus species isolated from fermented Uttapam batter. Biosci. Biotechnol. Res. Asia 9: 417–421 (2012)
Torriani S, Felis G, Dellaglio F. Differentiation of Lactobacillus plantarum, L. pentosus, and L. paraplantarum by recA gene sequence analysis and multiplex PCR assay with recA gene-derived primers. Appl. Environ. Microb. 67: 3450–3454 (2001)
Anastasio M, Pepe O, Cirillo T, Palomba S, Blaiotta G, Villani F. Selection and use of phytate-degrading LAB to improve cereal-based products by mineral solubilisation during dough fermentation. J. Food Sci. 75: 28–35 (2010)
Subrota H, Shilpa V, Brij S, Vandna K, Surajit M. Antioxidative activity and polyphenol content in fermented soy milk supplemented with WOC-70 by probiotic lactobacilli. Int. Food Res. J. 20: 2125–2131 (2013)
Wilkens W, Mattick L, Arid Hand D. Effect of processing method on oxidative off flavors of soybean milk. Food Technol.-Chicago 21: 86–90 (1967)
Sadasivam S, Manickam A. Biochemical Methods. New Age Int. Publishers, New Age Int. (P) Ltd., New Delhi, India. pp. 215–216 (2008)
Claudia A, Graciela E, Ferraro GE, Rosana F. Total polyphenol content and antioxidant capacity of commercially available tea (Camellia sinensis) in Argentina. J. Agr. Food chem. 56: 9225–9229 (2008)
Aijie L, Shouwei Y, Li L. Structure, trypsin inhibitor activity and functional properties of germinated soybean protein isolate. Int. J. Food Sci. Tech. 49: 911–919 (2014)
Moraes P, Perin L, Ortolani M, Yamazi A, Vicosa G, Nero L. Protocols for the isolation and detection of lactic acid bacteria with bacteriocinogenic potential. LWT-Food Sci. Technol. 43: 1320–1324 (2010)
Reale A, Konietzny U, Coppola R, Sorrentino E, Greiner R. The importance of lactic acid bacteria for phytate degradation during cereal dough fermentation. J. Agr. Food Chem. 55: 2993–2997 (2007)
Tang AL, Wilcox G, Walker K, Shah N, Ashton J, Stojanovska L. Phytase activity from Lactobacillus spp. in calcium-fortified soymilk. J. Food Sci. 75: 373–376 (2010)
Zohreh K, Mahboobeh MN, Mohammad HN, Mahdi G, Hillary D, Anna MS. Phytase activity of lactic acid bacteria isolated from dairy and pharmaceutical probiotic products. Int. J. Enteric. Pathog. 1: 12–16 (2013)
Lin MY, Yen CL. Antioxidative ability of lactic acid bacteria. J. Agr. Food Chem. 47: 1460–1466 (1999)
Rekha C, Vijayalakshmi G. Biomolecules and nutritional quality of soymilk fermented with probiotic yeast and bacteria. Appl. Biochem. Biotech. 151: 452–463 (2008)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sadishkumar, V., Kolanchiammal, R. & Jeevaratnam, K. The effect of Piper betle leaves on lacto-fermentation of idli batter, characterization and applicability of potent isolates in soymilk fermentation. Food Sci Biotechnol 24, 2137–2144 (2015). https://doi.org/10.1007/s10068-015-0284-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-015-0284-8