Abstract
Objectives
We aimed to present the demographic, clinical, laboratory, and treatment data of children with non-infectious uveitis and to evaluate the risk factors for the development of complications and the need for biological treatment.
Method
Patients diagnosed with non-infectious uveitis in childhood and followed up for at least 1 year were included in the study. Demographic data, including age, gender, age at diagnosis, uveitis in first-degree relatives, and rheumatologic diseases, were obtained retrospectively from medical records. The presence of complications or the need for biologic therapy was considered a composite outcome suggesting severe disease.
Results
The study included 123 patients (female: n = 59, 48%). The mean age at diagnosis was 14.89 ± 4.86 years. Uveitis was symptomatic in 104 patients (84.6%). Approximately one-quarter of the patients had at least one rheumatic disease (n = 35, 28.5%), the most common being juvenile idiopathic arthritis. Thirty-three patients (26.8%) had anti-nuclear antibody positivity. Biologic agents were needed in 60 patients (48.8%). Complications developed in 14 patients (11.4%). Early age at disease onset (aOR, 0.875; 95% C.I. 0.795–0.965, p = 0.007) and female gender (aOR, 2.99; 95% C.I. 1.439–6.248, p = 0.003) were significantly associated with the need for biologic treatment, while Behçet’s disease (BD) was strongly associated with uveitis-related complications (aOR, 14.133; 95% C.I. 2.765–72.231, p = 0.001).
Conclusion
We suggest that among pediatric patients with non-infectious uveitis, females, those with an early age of disease onset, and those with BD need to be closely monitored due to a significantly increased risk of severe disease.
Key Points • Limited data exist on the clinical course of non-infectious uveitis in children and the associated risk factors for severe disease. • Our study reveals that nearly a quarter of pediatric patients with non-infectious uveitis also have a rheumatic disease. • Among pediatric patients diagnosed with non-infectious uveitis, we observed an increased risk of severe disease in those with an earlier onset age, in female patients, and in those diagnosed with Behçet’s disease. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pediatric uveitis is a serious and rare condition characterized by inflammation of the uveal components of the eye, including the iris, ciliary body, and retina [1]. Idiopathic uveitis accounts for 65–90% of all pediatric uveitis cases [2, 3]. Pediatric uveitis may be associated with various systemic diseases such as juvenile idiopathic arthritis (JIA), Behçet’s disease (BD), tubulointerstitial nephritis uveitis (TINU), sarcoidosis, and vasculitis [4,5,6]. JIA is the most common systemic disease associated with cases of pediatric uveitis that cannot be attributed to an infectious cause [7].
The main symptoms of uveitis are pain, redness, photophobia, and blurred vision [8]. Because uveitis in children is often insidious and children are less likely to express themselves, the diagnosis of the disease is delayed [8]. As a consequence of delayed diagnosis, uveitis is more likely to lead to complications such as cataracts and band keratopathy in children than in adults [9, 10].
Treatment of uveitis typically includes topical or systemic corticosteroids and conventional disease-modifying antirheumatic drugs (cDMARDs). Biologic therapies have proven to be effective in patients who cannot be controlled with conventional treatments [9].
Limited research has been conducted on non-infectious uveitis in children, and the number of patients included in previous studies was relatively small. The primary aim of our study was to analyze the demographic, laboratory, and systemic disease characteristics of a large pediatric patient population with non-infectious uveitis. Additionally, we aimed to investigate the treatments administered, patients’ responses to drugs, and complications associated with uveitis. Our secondary goal was to identify potential risk factors that may predict the need for biological treatment or the likelihood of experiencing uveitis-related complications.
Materials and methods
Patients and data collection
Our study comprised individuals diagnosed with non-infectious uveitis before the age of 21, who experienced the first uveitis attack before the age of 18, and had a minimum of 1 year of follow-up duration. This criterion was based on the guidelines and arrangements in our country that allow us to follow-up these patients until they reach the age of 21.
The demographic, clinic, and laboratory data were obtained from the pediatric rheumatology department’s medical records retrospectively. The ophthalmology department in same center, which specializes in uveitis, provided the data containing eye examination findings. Anatomical classification of uveitis and disease course (acute, chronic, or remission) were classified according to Standardization of Uveitis Nomenclature (SUN) [11]. Underlying systemic diseases, extraocular findings, treatments prescribed for uveitis, complications, and laboratory findings at admission including antinuclear antibody (ANA), human leukocyte antigen-27 (HLA-B27), rheumatoid factor (RF), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and platelet count (PLT) were also recorded.
The diagnosis of JIA was made according to the International League of Rheumatology Societies (ILAR) diagnostic criteria for JIA [12]. Pediatric Behçet’s diagnostic criteria were used to make the diagnosis of BD [13]. The diagnoses of familial Mediterranean fever (FMF) and non-hereditary, periodic fever, aphthous, pharyngitis, and adenitis (PFAPA) were made according to the Eurofever/PRINTO clinical classification criteria for PFAPA and hereditary recurrent fevers [14]. The diagnosis of sarcoidosis and tubulointerstitial nephritis and uveitis (TINU) was based on clinical, laboratory, screening, and renal biopsy findings [15, 16].
This study was conducted in compliance with the Helsinki Declaration as well as local laws and regulations. Because of the retrospective nature of the study, obtaining written informed consent from the patients was not required. Ethics committee approval (date/no.: 13/12/23–2023/0919) was obtained from the Istanbul Medeniyet University, Ethics Committee of Clinical Trials.
Terms and definitions
Non-infectious uveitis was defined as patients not affected by infectious diseases that can cause uveitis, such as toxoplasmosis, toxocariasis, tuberculosis, and viral infections (e.g., herpes simplex virus or cytomegalovirus) [17]. The presence of complications including cataract, synechia, glaucoma, and band keratopathy or the need for biological treatment was considered a composite outcome, and the patients with this composite outcome were considered to have severe disease.
Statistical analysis
The statistical analysis was executed using SPSS for Windows, version 26.0 (SPSS Inc., Chicago, IL). The continuous variables were exhibited as either the mean ± standard deviation or the median (minimum–maximum), depending on their distribution, which was established using the Kolmogorov–Smirnov test. The assessment of categorical variables was carried out using the chi-square test or Fisher’s exact test when applicable. The associations between continuous variables and categoric variables were assessed using the Mann–Whitney U test when abnormally distributed and Student’s t tests when normally distributed.
The risk factors of biological requirement and uveitis-related complications identified as the risk factors for severe disease were assigned by using binary logistic regression analysis for both. While model 1 was set for biological requirement, model 2 was for uveitis-related complications. The variables that were found to be significant in comparing tests and confounding factors were included in these models. Gender, presence of rheumatologic disease, ANA positivity, and uveitis type were considered to be confounding factors. Since all BD was panuveitis, uveitis type was not considered a confounding factor in model 2.
Omnibus test and Hosmer–Lemeshow tests were used as model fitting test for binary logictic regression analysis.The significance level employed to determine statistical significance was set at p < 0.05, and Prism software (version 8, GraphPad Software, San Diego, California) was utilized to analyze and graph the data.
Results
Demographic, laboratory, and underlying systemic diseases data
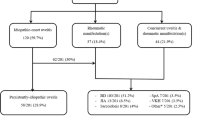
The average age of 123 patients with non-infectious uveitis was 14.89 ± 4.86 years and 48% (n = 59) was female. The median age at onset of uveitis symptoms was 11.72 (1.96–20.01) years. Uveitis was symptomatic in 104 (84.6%) of the patients in our cohort. Bilateral uveitis was more common than unilateral uveitis (82.1% vs 17.9%). According to the anatomical localization of uveitis, 97 patients (78.9%) had anterior uveitis, 11 (8.9%) had intermediate uveitis, 1 (0.8%) had posterior uveitis, and 14 (11.4%) had panuveitis. ANA was positive in 33 (26.8%) of the patients. The median CRP, and the median erythrocyte sedimentation rate (ESR) levels were 2.71 (0.08–94) mg/L and 11 (2–91) mm/hour at admission, respectively. Nearly a quarter of patients (n = 35, 28,5%) had at least one type of rheumatic disease, and the most common one was JIA (n = 19, 15.4%). Detailed data are given in Table 1.
Treatment options and uveitis-related complications
All patients in our study received steroid eye drops and 68.3% (n = 84) also received systemic steroid treatment. Following the diagnosis of uveitis, 78.9% (n = 97) of the patients were treated with cDMARD. The most preferred cDMARD was methotrexate (71.5%). 48.8% of patients (n = 60) received biologic treatment. The most preferred biological DMARDs (bDMARDs) were tumor necrosis factor-α (TNF-α) blockers with adalimumab most commonly prescribed (45.5%). In 26 patients (21.1%), neither cDMARD nor bDMARD treatment was required, and uveitis attacks could be controlled with topical steroids only. Almost one tenth of the patients (11.4%, n = 14) of the patients experienced uveitis-related complications, and the most common was cataract (n = 10). Only three patients required surgical intervention. Detailed data are available in Table 1.
Comparisons between the patients with and without complications
The uveitis disease onset was significantly earlier in the patients who experienced complications (10.37 (2.92–16.91) years vs. 11.80 (1.96–20.01) years; p = 0.013). Furthermore, BD was significantly more common in the patients with complications (28.6% vs. 2.8%; p < 0.001). While 4 of 7 patients with BD and 2 of 19 patients with JIA developed complications, none of the patients with other rheumatic disease had complications. Detailed data for both groups are presented in Table 2.
Comparative analysis of the patients who needed biologic treatments and who did not
The need for biologic therapy was significantly higher in females as shown in Fig. 1 (61.7% vs. 34.9%; p = 0.003) and those requiring biologic therapy had a significantly younger age at onset of uveitis (10.57 (1.96–16.9) years vs. 12.56 (2.82–20.01) years; p = 0.013).Vascular and respiratory involvement signs were only observed in biologic group. Although the most common diagnosis among the biologic receivers was JIA, patients with BD had the highest percentage of patients who needed biologics. In the follow-up, ocular complications were significantly more common in patients who needed biologic treatments. Comparative analysis of both groups are presented in Table 3.
Comparison of age at disease onsets and gender frequencies of the patients according to biologic needing and ocular complication development. A Comparison of age at disease onsets between the patients required biological treatment and the patients did not. B Comparison of age at disease onsets between the patients experienced ocular complication and the patients did not. C Comparison of gender frequencies between the patients required biological treatment and the patients did not. D Comparison of gender frequencies between the patients experienced ocular complication and the patients did not
Risk factors of severe disease
Female gender (univariate: aOR 2.99, 95% C.I. 1.439–6.248, p = 0.003; multivariate: aOR 3.265, 95% C.I. 1.455–7.322, p = 0.004) and younger age at uveitis onset (univariate: aOR 0.875, 95% C.I. 0.795–0.965, p = 0.007; multivariate: aOR 0.846, 95% C.I. 0.759–0.944, p = 0.007) were strongly associated with increased need for biologics in both univariate and multivariate logistic regression analysis. Furthermore, BD was a strong risk factor for complications in both univariate (aOR, 14.133; 95% C.I. 2.765–72.231, p = 0.001) and multivariate (aOR 24.159, 95% C.I. 2.592–225,152, p = 0.005) logistic regression analysis (Table 4).
Discussion
In this study, we analyzed the demographic and laboratory data, underlying systemic diseases, treatments administered, and uveitis-related complications experienced by our patients with non-infectious pediatric uveitis. Our study found that being female and early age at disease onset were significantly associated with an increased need for biologic treatments. Additionally, BD was strongly linked to uveitis-related complications. To build upon these findings, it is essential to delve deeper into the specific treatments and their outcomes, examining how these factors contribute to managing the disease and improving patient prognosis.
It is widely recognized that female gender is a risk element for JIA-related uveitis [17]. Nevertheless, the prevalence of childhood non-infectious uveitis among different genders remains ambiguous, as various studies have reported contradictory findings [18,19,20,21]. The male/female ratio in our cohort was 1.08:1 (64 male and 59 female). We attribute the balanced gender distribution in our cohort to the relatively low JIA rate. Previous pediatric uveitis studies of the gender distribution support to our study [20,21,22]. In a study conducted by Ozdal et al. [23], it was found that the higher prevalence of male gender was linked to the fact that BD was the most common comorbid systemic disease, which has been reported that BD is slightly more common in male children [24].
The most common type was anterior uveitis which was seen in almost four-fifth of the patients, consistent with the previous reports [22, 23]. Approximately one quarter of our patients had an underlying systemic rheumatologic disease. The most common rheumatologic disease was JIA, which was largely consistent with previous studies [19, 21, 25]. It is known that uveitis associated with JIA most commonly causes anterior uveitis [8, 26]. In approximately 80% of JIA patients in our cohort, the type of uveitis was anterior uveitis, which was consistent with the previous literature [26, 27]. However, it is important to note that the majority of patients with anterior uveitis do not have JIA. We suspect that some of these cases may be JIA cases that started with ocular involvement but did not have the chance to develop arthritis due to the frequent use of cDMARD and biologic drugs, which are also used in the treatment of arthritis in this patient group.
All our patients with BD had panuveitis. This data confirms the knowledge that Behçet’s disease-associated uveitis is typically characterized by panuveitis [17]. We also found that around 40% of our patients with BD did not report symptomatic disease. This might be related with the limited ability of the children to notice or report their ocular problems, unlike adults with the same disease. Therefore, we think that even if they have no ocular complaints, uveitis screening in patients with BD, as well as JIA, is crucial to capture these cases.
Although it is rare, uveitis was reported to be the second most common ocular involvement sign after the conjunctivitis in FMF patients [28]. One of our patients had with asymptomatic anterior uveitis had coexisting FMF and JIA. It remains uncertain whether the risk of uveitis changes in JIA with coexisting FMF. Besides, uveitis associated with PFAPA syndrome has been reported rarely. However, the exact frequency and mechanism are not clearly known [29]. One of our patients with isolated PFAPA had acute symptomatic anterior uveitis. Our findings are in line with the previously reported data that autoinflammatory diseases may be accompanied by red eye including conjunctivitis, episcleritis, and uveitis [30].
In our study, we found that patients with rheumatic disease had significantly higher ESR levels at presentation compared to patients with isolated uveitis. Although not statistically significant, patients with rheumatic disease also had higher CRP levels at presentation. In a study comparing patients with isolated uveitis, BD-related uveitis, and JIA-related uveitis, ESR values were found to be significantly higher in patients with rheumatologic diseases, similar to our data [20]. Consequently, we suggest that pediatric uveitis patients with high ESR values should undergo thorough examination for potential underlying rheumatologic diseases.
It is known that oligoarticular type and ANA positivity are risk factors for the development of uveitis in JIA patients [17]. Consistent with the literature data, we found that ANA was positive in almost half of the patients with JIA-related uveitis. In addition, approximately 2/3 of the ANA positive JIA patients had oligoarticular JIA.
Although all patients in our study received local glucocorticoids as first-line treatment, only in one fifth of our patient’s uveitis could be controlled with local treatment. The remaining patients needed cDMARDs. The most commonly prescribed cDMARD was methotrexate (MTX) and azathioprine (AZA) was the second one, similar to previous studies [20, 21]. Biologic therapy was needed in almost half of our patients. Anti-TNFs are the most commonly used biologic treatment agents in accordance with recommendations of treatment guidelines [9, 10].
To the best of our knowledge, this study demonstrated for the first time that the need for biologic treatments was significantly higher in female patients compared to male pediatric uveitis patients. Furthermore, the results of the binary logistic regression analysis indicated that being female was a risk factor for the necessity of biological treatment. It is known that females are at increased risk of autoimmune diseases and JIA-related uveitis [17, 26, 31]. Our analysis indicated that patients with rheumatologic diseases had a more pressing need for biological treatment compared to those with idiopathic uveitis. However, in the group with rheumatologic disease, there were 19 female and 16 male patients, with the numbers being relatively equal. Additionally, although we added the presence of rheumatic disease in to the regression analysis which was set for evaluate the risk factors for biologic requirement as a confounding factor, we found the female gender as an independent risk factor. Therefore, we suggest that female patients with uveitis should be closely monitored due to significantly increased biological treatment needing.
We showed that early disease onset is also a significant predictor of biological treatment requirement, as supported by previous [32]. Earlier disease onset may be due to a higher genetic burden that promotes autoimmune inflammation. These patients also have a longer exposure to chronic inflammation, which can transform into a form that is not easily controlled. Therefore, we think that biological treatment options may be considered in early stages of the diseases in patients with younger age of disease onset.
Pediatric uveitis is a condition that can result in a variety of complications, including cataract, synechiae, and glaucoma. The prevalence of these complications varies significantly between studies, ranging from 14.7% to 69% [18, 33,34,35]. In our study, 11.4% of the patients experienced complications, and the most common was cataract. The main factors that cause this variation in complication rated can be different distribution of systemic diseases in different cohorts, variations in disease duration, and easier access to biologic treatments.
The influence of gender was not thoroughly studied in pediatric uveitis patients. Here, we found a higher rate of uveitis-related complications in girls compared to boys; the complication rate in BD was also significantly higher and not affected by the confounding factors we identified. BD is typically characterized by bilateral, recurrent panuveitis [17]. Posterior segment involvement is associated with a higher risk of ocular complications, and all pediatric BD patients in our cohort had panuveitis similar to previous reports [12, 36]. Therefore, it is not surprising that this patient group had a higher complication rate. Our study additionally shows that BD is a significant risk factor for the development of ocular complications.
The main limitation of our study is that it was designed as a retrospective single-center study. Additionally, despite having a reasonable number of patients overall, we did not have a sufficient number of patients in the subgroups to conclude uveitis associated with rare rheumatologic diseases. Another noteworthy limitation of our study was that the follow-up period of at least 1 year may be inadequate to evaluate the progression of chronic conditions. On the other hand, the main strength of our study is that we evaluated a broad range of rheumatic diseases and analyzed the possible predictors of the need for biological treatments, complications, and risk factors for severe disease.
In conclusion, we found that an early age of uveitis onset and female gender are strongly associated with the need for biologic treatment, while BD is a strong predictor of uveitis-related complications. We think that female gender, those with early onset of disease, and patients with BD should be closely monitored due to the significantly increased risk of serious illness, and more caution should be exercised when prescribing treatment in these patients.
Data availability
All data relevant to the study are included in the article.
References
Maccora I, Sen ES, Ramanan AV (2020) Update on noninfectious uveitis in children and its treatment. Curr Opin Rheumatol 32(5):395–402. https://doi.org/10.1097/BOR.0000000000000723
BenEzra D, Cohen E, Maftzir G (2005) Uveitis in children and adolescents. Br J Ophthalmol 89(4):444–448. https://doi.org/10.1136/BJO.2004.050609
Kump LI, Cervantes-Castañeda RA, Androudi SN, Foster CS (2005) Analysis of pediatric uveitis cases at a tertiary referral center. Ophthalmology 112(7). https://doi.org/10.1016/j.ophtha.2005.01.044
Angeles-Han ST, Lo MS, Henderson LA, Lerman MA, Abramson L, Cooper AM, Parsa MF, Zemel LS, Ronis T, Beukelman T, Cox E, Sen HN, Holland GN, Brunner HI, Lasky A, Egla Rabinovich C (2019) Childhood arthritis and rheumatology research alliance consensus treatment plans for juvenile idiopathic arthritis-associated and idiopathic chronic anterior uveitis. Arthritis Care Res 71(4):482–491. https://doi.org/10.1002/ACR.23610
Al-Haddad C, Boughannam A, Abdul Fattah M, Tamim H, el Moussawi Z, Hamam, RN (2019) Patterns of uveitis in children according to age: comparison of visual outcomes and complications in a tertiary center. BMC Ophthalmol 19(1). https://doi.org/10.1186/S12886-019-1139-5
Ruperto N, Ozen S, Pistorio A, Dolezalova P, Brogan P, Cabral DA, Cuttica R, Khubchandani R, Lovell DJ, O’Neil KM, Quartier P, Ravelli A, Iusan SM, Filocamo G, Magalhães CS, Unsal E, Oliveira S, Bracaglia C, Bagga A, Martini A (2010) EULAR/PRINTO/PRES criteria for Henoch-Schönlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008. Part I: Overall methodology and clinical characterisation. Ann Rheum Dis 69(5):790–797. https://doi.org/10.1136/ARD.2009.116624
Sen ES, Ramanan AV (2020) Juvenile idiopathic arthritis-associated uveitis. Clin Immunol (Orlando, Fla.) 211. https://doi.org/10.1016/J.CLIM.2019.108322
Reiff A, Kadayifcilar S, Özen S (2013) Rheumatic inflammatory eye diseases of childhood. Rheum Dis Clin North America 39(4). https://doi.org/10.1016/j.rdc.2013.05.005
Angeles-Han ST, Ringold S, Beukelman T, Lovell D, Cuello CA, Becker ML, Colbert RA, Feldman BM, Holland GN, Ferguson PJ, Gewanter H, Guzman J, Horonjeff J, Nigrovic PA, Ombrello MJ, Passo MH, Stoll ML, Rabinovich CE, Sen HN, … Reston J (2019) 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Screening, Monitoring, and Treatment of Juvenile Idiopathic Arthritis-Associated Uveitis. Arthritis Care Res 71(6):703–716. https://doi.org/10.1002/ACR.23871
Constantin T, Foeldvari I, Anton J, de Boer J, Czitrom-Guillaume S, Edelsten C, Gepstein R, Heiligenhaus A, Pilkington CA, Simonini G, Uziel Y, Vastert SJ, Wulffraat NM, Haasnoot AM, Walscheid K, Pálinkás A, Pattani R, Györgyi Z, Kozma R, … Ramanan AV (2018) Consensus-based recommendations for the management of uveitis associated with juvenile idiopathic arthritis: the SHARE initiative. Ann Rheum Dis 77(8):1107–1117. https://doi.org/10.1136/ANNRHEUMDIS-2018-213131
Jabs DA, Nussenblatt RB, Rosenbaum JT, Atmaca LS, Becker MD, Brezin AP, Chee SP, Davis JL, Deschenes J, de Smet M, Dick A, Dunn JP, Forrester JV, Franklin RM, Godfrey WA, Gold-Stein DA, Graham EM, Herbort CP, Holland GN, … Rothova A (2005) Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol 140(3):509–516. https://doi.org/10.1016/J.AJO.2005.03.057
Martini A, Ravelli A, Avcin T, Beresford MW, Burgos-Vargas R, Cuttica R, Ilowite NT, Khubchandani R, Laxer RM, Lovell DJ, Petty RE, Wallace CA, Wulffraat NM, Pistorio A, Ruperto N (2019) Toward new classification criteria for juvenile idiopathic arthritis: first steps, Pediatric Rheumatology International Trials Organization International Consensus. J Rheumatol 46(2):190–197. https://doi.org/10.3899/JRHEUM.180168
Koné-Paut I, Shahram F, Darce-Bello M, Cantarini L, Cimaz R, Gattorno M, Anton J, Hofer M, Chkirate B, Bouayed K, Tugal-Tutkun I, Kuemmerle-Deschner J, Agostini H, Federici S, Arnoux A, Piedvache C, Ozen S, Retornaz K, Jurquet AL, … Tran TA (2016) Consensus classification criteria for paediatric Behçet’s disease from a prospective observational cohort: PEDBD. Ann Rheum Dis 75(6):958–964. https://doi.org/10.1136/ANNRHEUMDIS-2015-208491
Gattorno M, Hofer M, Federici S, Vanoni F, Bovis F, Aksentijevich I, Anton J, Arostegui JI, Barron K, Ben-Cherit E, Brogan PA, Cantarini L, Ceccherini I, de Benedetti F, Dedeoglu F, Demirkaya E, Frenkel J, Goldbach-Mansky R, Gul A, … Ruperto N (2019) Classification criteria for autoinflammatory recurrent fevers. Ann Rheum Dis 78(8):1025–1032. https://doi.org/10.1136/ANNRHEUMDIS-2019-215048
Herbort CP, Rao NA, Mochizuki M (2009) International criteria for the diagnosis of ocular sarcoidosis: results of the first International Workshop On Ocular Sarcoidosis (IWOS). Ocul Immunol Inflamm 17(3):160–169. https://doi.org/10.1080/09273940902818861
MacKensen F, Billing H (2009) Tubulointerstitial nephritis and uveitis syndrome. Curr Opin Ophthalmol 20(6):525–531. https://doi.org/10.1097/ICU.0B013E3283318F9A
Tuğal-Tutkun İ (2023) An overview of pediatric uveitis. Turkish Archives of Pediatrics 58(4):363–370. https://doi.org/10.5152/TurkArchPediatr.2023.23086
Ozdel S, Baglan E, Gungor T, Yazılıtas F, Cakıcı EK, Ozdal PC, Bulbul M (2021) Comparison of pediatric patients with noninfectious idiopathic uveitis and noninfectious uveitis associated with an underlying systemic disease: from a referral center in Turkey. Postgrad Med 133(4):444–448. https://doi.org/10.1080/00325481.2021.1902684
Smith JA, Mackensen F, Sen HN, Leigh JF, Watkins AS, Pyatetsky D, Tessler HH, Nussenblatt RB, Rosenbaum JT, Reed GF, Vitale S, Smith JR, Goldstein DA (2009) Epidemiology and course of disease in childhood uveitis. Ophthalmology 116(8). https://doi.org/10.1016/J.OPHTHA.2009.05.002
Gezgin Yıldırım D, Hasanreisoğlu M, Bakkaloğlu SA (2023) Comparison of pediatric patients with idiopathic uveitis, and uveitis due to juvenile idiopathic arthritis and Behçet’s disease. Postgrad Med 135(1):79–85. https://doi.org/10.1080/00325481.2022.2133853
Tekin ZE, Yener GO, Akbulut S, Çetin EN, Yüksel S (2021) Follow-up findings of non-infectious pediatric uveitis patients. Turk J Ophthalmol 51(6):351–357. https://doi.org/10.4274/TJO.GALENOS.2021.38585
Shin Y, Kang JM, Lee J, Lee CS, Lee SC, Ahn JG (2021) Epidemiology of pediatric uveitis and associated systemic diseases. Pediatr Rheumatol Online J 19(1). https://doi.org/10.1186/S12969-021-00516-2
Ozdal PÇ, Sen E, Yazici A, Ozturk F (2012) Patterns of childhood-onset uveitis in a referral center in Turkey. J Ophthalmic Inflamm Infect 2(1):13. https://doi.org/10.1007/S12348-011-0044-8
Çirkinoğlu MS, Demir S, Bilginer Y, Özen S (2019) Behçet’s disease in children: single-center experience. Turk Arch Pediatr/Türk Pediatri Arşivi 54(3):179. https://doi.org/10.14744/TURKPEDIATRIARS.2019.15045
Çakan M, Yildiz Ekıncı D, Gül Karadağ Ş, Aktay Ayaz N (2019) Etiologic spectrum and follow-up results of noninfectious uveitis in children: a single referral center experience. Arch Rheumatol 34(3):294–300. https://doi.org/10.5606/ARCHRHEUMATOL.2019.7253
Tugal-Tutkun I (2011) Pediatric uveitis. J Ophthalmic Vis Res 6(4):259–69
Yalçındağ FN, Özdal P, Özyazgan Y, Batıoğlu F, Tugal-Tutkun I (2023) Pediatric uveitis in Turkey: the National Registry Report II. Ocul Immunol Inflamm 31(10):1971–1977. https://doi.org/10.1080/09273948.2022.2110900
Avar-Aydin PO, Cakar N, Ozcakar ZB, Yalcindag N, Yalcinkaya F (2022) Ocular inflammatory diseases in children with familial Mediterranean fever: a true association or a coincidence? Int Ophthalmol 42(4):1249–1257. https://doi.org/10.1007/S10792-021-02111-6
Choi RY, Shakoor A, Bohnsack J, Vitale AT (2019) Intermediate uveitis associated with periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis syndrome. Retin Cases Brief Rep 13(4):367–370. https://doi.org/10.1097/ICB.0000000000000600
Yıldız M, Haşlak F, Adrovic A, Barut K, Kasapçopur Ö (2020) Autoinflammatory diseases in childhood. Balkan Med J 37(5):236. https://doi.org/10.4274/BALKANMEDJ.GALENOS.2020.2020.4.82
Kesen MR, Setlur V, Goldstein DA (2008) Juvenile idiopathic arthritis-related uveitis. Int Ophthalmol Clin 48(3):21–38. https://doi.org/10.1097/IIO.0B013E31817D998F
Osswald D, Rameau AC, Terzic J, Sordet C, Bourcier T, Sauer A (2022) Risk factors leading to anti-TNF alpha therapies in pediatric severe uveitis. Front Pediatr 10. https://doi.org/10.3389/FPED.2022.802977
Sahin S, Acari C, Sonmez HE, Kilic FZ, Sag E, Dundar HA, Adrovic A, Demir S, Barut K, Bilginer Y, Sozeri B, Unsal E, Ozen S, Kasapcopur O (2021) Frequency of juvenile idiopathic arthritis and associated uveitis in pediatric rheumatology clinics in Turkey: a retrospective study, JUPITER. Pediatr Rheumatol Online J 19(1). https://doi.org/10.1186/S12969-021-00613-2
Paroli MP, Spinucci G, Liverani M, Monte R, Pezzi PP (2009) Uveitis in childhood: an Italian clinical and epidemiological study. Ocul Immunol Inflamm 17(4):238–242. https://doi.org/10.1080/09273940802702561
Morelle G, Gueudry J, Uettwiller F, Wouters C, Bader-Meunier B, Robert MP, Monnet D, Bodaghi B, Grall-Lerosey M, Quartier P (2019) Chronic and recurrent non-infectious paediatric-onset uveitis: a French cohort. RMD Open 5(2). https://doi.org/10.1136/RMDOPEN-2019-000933
Pivetti-Pezzi P, Accorinti M, Abdulaziz M, Cava M, Torella M, Riso D (1995) Behçets disease in children. Jpn J Ophthalmol 39(3):309–14
Acknowledgements
Paperpal, an AI language model, was employed for language editing.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
LK, OT, FE, EK, FK, ZA, FH, and KO were responsible for data collection and analysis. They all contributed to the writing of the manuscript, and reviewed and revised the manuscript. They all approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by Institutional Review Board (13/12/23–2023/0919) of Istanbul Goztepe Suleyman Yalcin City Hospital.
Informed consent
A written informed consent was obtained from all the participants included in this study and no identifying information of any participant was included in this paper.
The patient and public involvement statement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Prepublication note
None.
Disclosures
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Koru, L., Esen, F., Turkyilmaz, O. et al. Clinical characteristics of pediatric noninfectious uveitis and risk factors for severe disease: a single-center study. Clin Rheumatol (2024). https://doi.org/10.1007/s10067-024-07072-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10067-024-07072-6