Abstract
Background
The COVID-19 outbreak has led to the rapid development and administration of the COVID-19 vaccines worldwide. Data about the immunogenicity and adverse effects of the vaccine on patients with systemic autoimmune rheumatic diseases (SARDs) is emerging.
Aim
To evaluate Pfizer/BioNTech (BNT162b2) mRNA-based vaccine second-dose immunogenicity and safety, and the relation between them, in patients with SARDs.
Methods
A total of one hundred forty tow adults who received two doses of the BNT162b2 vaccine were included in the study. The SARDs group included Ninety-nine patients and the control group (forty-three participants) comprised a mixture of healthy participants and patients who were seen at the rheumatology clinic for non-SARDs. Anti-SARS-CoV-2 IgG antibodies against the Spike protein were evaluated using a SARS-CoV-2 IgG immunoassay. A level of > 150 AU/mL was considered positive. An adverse effects questionnaire was given to the participants upon their first visit to the clinic after their BNT162b2 vaccination.
Results
Of the 142 participants, 116 were seropositive (81.7%) and 26 (18.3%) were seronegative. Of the seronegative participants, 96.2% were SARDs patients. The proportion of seropositivity in the SARDs patients treated with any immunosuppressant was significantly lower (69.9%) compared to the control group and SARDs patients not receiving immunosuppressants (96.8%). A significant negative correlation between seronegativity and treatment with rituximab, mycophenolate mofetil (MMF), and prednisone was found in the SARDs group (p = 0.004, 0.044, 0.007 respectively). No fever was observed following the BNT162b2 vaccine in seronegative patients, and the frequency of musculoskeletal adverse effects upon the second dose of the BNT162b2 vaccine was significantly higher in seropositive compared to seronegative patients and in the control group compared to the SARDs patients (p = 0.045, p = 0.02 respectively).
Conclusion
A decline in the immunogenicity to the second dose of BNT162b2 mRNA is seen in patients with SARDs, especially in patients treated with rituximab, MMF, and prednisone. Adverse effects of the vaccine including fever and musculoskeletal symptoms might be a signal for the acquisition of immunity in those patients.
Key Points
• BNT162b2 mRNA vaccine is less immunogenic in SARDs patients compared to the control group.
• Rituximab, prednisone, and mycophenolate mofetil significantly reduced immunogenicity to the vaccine.
• There is a correlation between immunogenicity and adverse effects of the vaccine.
Similar content being viewed by others
Introduction
The COVID-19 outbreak, accompanied by its unprecedented impact on people and society, led to the rapid development and administration of the COVID-19 vaccines worldwide. The BioNTech/Pfizer mRNA-based (BNT162b2) vaccine encodes a genetically modified SARS-CoV-2 spike S protein [1] that is reported to elicit high titers of neutralizing antibodies to the virus in the vast majority of the vaccinated individuals [2]. The COVID-19 vaccines clinical trials largely excluded patients with systemic autoimmune rheumatic diseases (SARDs) and/or patients on immunosuppressive treatments, raising concern regarding the efficacy and safety of the COVID-19 vaccine amongst these groups of patients [3,4,5].
The COVID-19 pandemic has particularly impacted the lives of people with SARDs. Immunosuppressive treatment and the presence of comorbidities have been associated with an increased risk of COVID-19 infection including hospitalization, and even death [6, 7]. In SARDs patients, the immune response is altered and varies with the type of immunomodulatory regimen and the vaccine used [8, 9]. Owing to the type and degree of medications, some patients with SARDs may have less protection from the vaccines. For example, methotrexate (MTX), mycophenolate mofetil (MMF), tofacitinib, and prednisone (≥ 10 mg/day) have been shown to attenuate the vaccine-induced response upon receiving other non-COVID-19 vaccines [8]. Since data derived from the usage of other non-COVID-19 vaccines might not translate to the novel vaccines developed for COVID-19 [10, 11], it is crucial to study this aspect.
COVID-19 vaccines immunogenicity is usually measured using the humoral IgG to spike ‘S’ protein [12]. In our study, we assess the serology of 142 participants with SARDs and a control group to evaluate the immunogenicity and safety of the BNT162b2 mRNA-based vaccine, and to describe the impact of the type and degree of the immunosuppressive treatments.
Materials and methods
Study cohort
SARDs patients from the Rheumatology Clinic at Meir medical center (Kfar Saba, Israel) were recruited for this study between April and June 2021. The control group comprised a mixture of healthy participants and patients who were seen at the rheumatology clinic for non-SARDs (e.g., osteoarthritis and fibromyalgia). Patients with a previously reported clinical diagnosis of COVID-19 and a positive PCR COVID-19 test were excluded from the study. The study was approved by the Institutional Review Board (IRB) of Meir Medical Center, registered under identifier 0056–21. All participants provided written informed consent for participation in the study.
Blood draw and serum separation
Blood was drawn and collected in a serum separator tube (BD Vacutainer, #365,328, BD, Franklin Lakes, NJ, USA) from the control group and SARDs patients upon their first clinic visit post the Pfizer-BioNTech (BNT162b2) mRNA-based vaccination. The samples were allowed to clot at room temperature for 30–60 min. Samples were centrifuged at 2000 RCF for 10 min at 4 °C and serum was collected into an Eppendorf tube and frozen at –80 °C until further use.
Humoral immunity of the vaccine
The presence of anti-SARS-CoV-2 IgG antibodies was evaluated using a SARS-CoV-2 IgG chemiluminescent microparticle immunoassay (CMIA) (Abbott, Sligo, Ireland) intended for detection of IgG antibodies to the receptor binding domain (RBD) of the S1 subunit of the spike protein of SARS-CoV-2 in the serum samples of the participants. Results are provided in arbitrary units (AU) per milliliter as defined by the manufacturer, ranging between 0 and 40,000 AU/mL for the anti-S antibodies. A level of > 150 AU/mL was considered positive [13].
Patient questionnaire
The survey was performed using a cross-sectional method to evaluate the adverse effects of BNT162b2 vaccination. The questionnaire was developed in Hebrew for the convenience of the Israeli participants. The questionnaires included demographic details (e.g. age, and sex), information regarding rheumatic disease and comorbidities, and immunosuppressive treatment. The section dealing with adverse effects included yes/no questions regarding symptoms that occurred within one week of each dose of vaccine administration. The adverse effects questionnaire included questions about local reaction to the injection, fever, general effects (fatigue, headache, and chills), musculoskeletal effects (arthralgia and myalgia), gastrointestinal effects, and others (lymphadenopathy, rash). The sampling technique used for the study is convenience sampling.
Statistics
Categorical variables were summarized as frequency and percentage. Continuous variables were evaluated for normal distribution using a histogram and reported as median and interquartile range (IQR). Chi-square test and Fisher’s exact test were used to compare categorical variables and Mann–Whitney test was applied to compare continuous variables. Multivariable logistic regression was used to study associations while controlling for other variables. All statistical tests were two-sided and p < 0.05 was considered statistically significant. SPSS software was used for all statistical analyses (IBM SPSS Statistics for Windows, Ver. 25, IBM Corp., Armonk, NY, USA, 2017).
Results
Study cohort
A total of 142 adult participants were included in the study. The SARDs group consisted of 99 SARDs patients (69.7%), all had stable disease, treated on an ambulatory basis. The control group consisted of 43 participants (30.3%), a mixture of healthy participants and patients who were seen at the rheumatology clinic for non-SARDs (e.g., osteoarthritis and fibromyalgia). All the participants were recruited after receiving two doses of the BNT162b2 vaccine. The median age was 56 years and females accounted for 76.1% of the cohort (n = 108). The most common rheumatic diagnosis was rheumatoid arthritis (RA) (n = 28, 28.3%) and systemic sclerosis (SSC) (n = 24, 24.2%) (Fig. 1, Table 1).
Seventy-nine patients from the SARDs group (79.8%) were on immunomodulatory/immunosuppressive therapy, including hydroxychloroquine (HCQ) (n = 23, 29.1%), MTX (n = 18, 22.8%), and tumor necrosis factor inhibitors (TNF-i) (n = 15, 19%) (Table 1).
Humoral immunity to the BNT162b2 vaccine
Out of the 142 participants, 116 were found to be seropositive and 26 were seronegative. No gender differences were found (p = 0.694). The seropositive participants were younger with median age of 52 years (IQR 39–67) compared to 67 years (IQR 55–72) in the seronegative participants (P = 0.004). Twenty-five out of the 99 SARDs patients (25.3%) were found to be seronegative compared to 1 out of 44 participants in the control group (2.3%) (p = 0.001) (Fig. 2A). A multivariable analysis adjusted for age and gender demonstrated the SARDs group was approximately nine times more likely to have a seronegative result (OR = 9.43, 95%CI 1.20–76.9, p = 0.033). The median IgG titer in the control group was 866 AU/mL, whereas in the SARDs group it was 562.8 AU/mL. In the SARDs group, the patients who were not treated with any immunosuppressant had significantly higher median IgG titers (1666.1 AU/ml) compared to the patients treated with immunosuppressants (493.9 AU/ml). Furthermore, only 69.6% of the treated SARDs patients were seropositive compared to 96.8% seropositivity in the control group combined with the untreated rheumatic patients (p < 0.0001) (Fig. 2B).
Serology results according to study group. A In the SARDS group 25.3% were seronegative (orange) compared to 2.3% in the control group, P value 0.001. B Comparing serology results regarding the use of immunosuppressive or immunomodulatory drugs. In the SARDS group 69.6% in the treated group were seropositive (blue) compared to 96.8% among untreated participants (healthy and rheumatic patients), P value < 0.0001. SARDS, systemic autoimmune rheumatic diseases; SARDS-T, SARDs patients treated with any immunosuppressive or immunomodulatory drugs
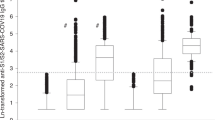
The correlation analysis depicts a significant negative correlation between the SARDs patients on rituximab, MMF, and prednisone treatment with seropositivity (p = 0.004, 0.044, 0.007 respectively) (Fig. 3, Table 2). Twenty-three out of the 79 treated SARDs patients were on HCQ treatment and significantly showed seropositivity (87%, p = 0.032).
Immunogenicity of the vaccine according to the use of immunosuppressive and immunomodulatory drugs. Prednisone, MMF, and mabthera were in negative correlation to seropositivity (p = 0.007, 0.044, 0.004, respectively). MTX, methotrexate; HCQ, hydroxychloroquine; SSZ, sulfasalazine; LEFL, leflunomide; COLCH, colchicine; MMF, mycophenolate mofetil; TNF-i, tumor necrosis factor inhibitors; IL6i, interleukin 6 inhibitors; JAKi, janus kinase inhibitors; MAB, mabthera; BELIM, belimumab; PRED, prednisone
Adverse effects associated with the BNT162b2 vaccination
Data on adverse events were collected based on the patients’ reports following each dose of the BNT162b2 vaccination. The frequency of the adverse reactions is shown in Table 3.
We compared between seropositive and seronegative participants (Table 3A) and between SARDs patients and the control group (Table 3B). General and Musculoskeletal adverse effects were more frequent in seropositive participants as well as in the control group. Fever after any or both doses of the BNT162b2 vaccine was more frequent in the control group (p = 0.04) and found only in seropositive participants (12.9% vs. 0%, p = 0.053).
SARDs patients treated with immunosuppressants had a trend towards a decrease in the frequency of fever upon the second dose of the BNT162b2 vaccine (p = 0.058) compared to the patients with no treatment. Only 4 patients (5.1%) from the 79 treated SARDs patients reported fever following any or both doses of the BNT162b2 vaccine compared to 11 (17.5%) patients that reported fever in the control group combined with SARDs patients who were not treated with immunomodulatory/immunosuppressive therapy (p = 0.017).
Discussion
This study of a cohort of 99 SARDs patients and 43 control participants was designed to test the hypothesis that humoral immune response and adverse reactions to SARS-CoV-2 mRNA-based vaccine might differ between SARDs patients who are treated with immunosuppressants and those who are not. This hypothesis was based on previous observation which showed reduced immunogenicity of the vaccine against SARS-CoV-2 when patients are treated with medications such as MMF, glucocorticoids and rituximab [11, 14].
Our results indicate that the BNT162b2 vaccine is less immunogenic in SARDs patients compared to the control group. Some of the medications used had a significant influence on the effectiveness of the BNT162b2 vaccine, while other medications such as cDMARDS and anti-cytokine biologics had no effect. We found that patients treated with rituximab, prednisone or MMF are less likely to develop antibodies against COVID-19 after receiving the BNT162b2 vaccine.
There are contradictory reports regarding the association between the humoral response to the BNT162b2 vaccine and adverse effects. For example, Grupper et al. [15] did not find a correlation between symptoms or severity of symptoms to humoral response, in their study groups, but Otani et al. [16] found an association between adverse effects and higher IgG levels. In our study, we found that fever and musculoskeletal complaints following the BNT162b2 vaccine are correlated with seropositivity.
In this study, fever, musculoskeletal, and general adverse effects were less frequent in the SARDs patients compared to the control group. Those adverse effects may reflect the acquisition of immunity to the vaccine against SARS-CoV-2, which immunosuppressive medications might blunt. These findings can help us reassure patients that the side effects they are experiencing are a sign for immunity to SARS-CoV-2. Furthermore, we should take the adverse effects in SARDs patients into consideration, and perhaps those who do not develop adverse events should be even more encouraged to receive a booster dose of the vaccine.
A major limitation of our study is the relatively small cohort with diverse rheumatic diseases and treatments and only a few patients treated with MMF and rituximab. Therefore, our analysis was limited and underpowered. Another limitation is the difference in age, demographic and medical history between patients and controls. However, difference in seropositivity was still significant in multivariate analysis adjusted for age and gender. The short follow-up time period after the second vaccination is another limitation. Additionally, it is still unclear to what extent the humoral response predicts the effectiveness of the vaccine over-time and what is the role of the cellular response in vaccine efficacy.
Conclusion
A decline in the immunogenicity to the second dose of BNT162b2 mRNA is seen in patients with SARDs, especially in patients treated with rituximab, MMF, and prednisone. Adverse effects of the vaccine including fever and musculoskeletal symptoms might be a signal for acquisition of immunity in those patients.
Change history
17 October 2022
A Correction to this paper has been published: https://doi.org/10.1007/s10067-022-06412-8
References
Dooling K, Gargano JW, Moulia D, Wallace M, Rosenblum HG, Blain AE et al (2021) Use of Pfizer-BioNTech COVID-19 vaccine in persons aged ≥16 years: recommendations of the advisory committee on immunization practices - United States, September 2021. MMWR Morb Mortal Wkly Rep 70(38):1344–1348
Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S et al (2020) Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 383(27):2603–2615
Hazlewood GS, Pardo JP, Barnabe C, Schieir O, Barber CEH, Bernatsky S et al (2021) Canadian rheumatology association recommendation for the use of COVID-19 Vaccination for patients with autoimmune rheumatic diseases. J Rheumatol 48(8):1330–1339
Curtis JR, Johnson SR, Anthony DD, Arasaratnam RJ, Baden LR, Bass AR et al (2021) American College of Rheumatology Guidance for COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases: Version 3. Arthritis Rheumatol (Hoboken, NJ) 73(10):e60-75
Geisen UM, Berner DK, Tran F, Sümbül M, Vullriede L, Ciripoi M et al (2021) Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Dis 80(10):1306–1311
Gianfrancesco M, Hyrich KL, Al-Adely S, Carmona L, Danila MI, Gossec L et al (2020) Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis 79(7):859–866
Strangfeld A, Schäfer M, Gianfrancesco MA, Lawson-Tovey S, Liew JW, Ljung L et al (2021) Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis 80(7):930–942
Papp KA, Haraoui B, Kumar D, Marshall JK, Bissonnette R, Bitton A et al (2019) Vaccination guidelines for patients with immune-mediated disorders on immunosuppressive therapies. J Cutan Med Surg 23(1):50–74
Rondaan C, Furer V, Heijstek MW, Agmon-Levin N, Bijl M, Breedveld FC et al (2019) Efficacy, immunogenicity and safety of vaccination in adult patients with autoimmune inflammatory rheumatic diseases: a systematic literature review for the 2019 update of EULAR recommendations. RMD Open 5(2):e001035
Prendecki M, Clarke C, Edwards H, McIntyre S, Mortimer P, Gleeson S et al (2021) Humoral and T-cell responses to SARS-CoV-2 vaccination in patients receiving immunosuppression. Ann Rheum Dis 80(10):1322–1329
Friedman MA, Curtis JR, Winthrop KL (2021) Impact of disease-modifying antirheumatic drugs on vaccine immunogenicity in patients with inflammatory rheumatic and musculoskeletal diseases. Ann Rheum Dis 80(10):1255–1265
Walsh EE, Frenck RWJ, Falsey AR, Kitchin N, Absalon J, Gurtman A et al (2020) Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med 383(25):2439–2450
Hagin D, Freund T, Navon M, Halperin T, Adir D, Marom R et al (2021) Immunogenicity of Pfizer-BioNTech COVID-19 vaccine in patients with inborn errors of immunity. J Allergy Clin Immunol 148(3):739–749
Furer V, Eviatar T, Zisman D, Peleg H, Paran D, Levartovsky D et al (2021) Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis 80(10):1330–1338
Grupper A, Katchman E, Ben-Yehoyada M, Rabinowich L, Schwartz D, Schwartz IF et al (2021) Kidney transplant recipients vaccinated before transplantation maintain superior humoral response to SARS-CoV-2 vaccine. Clin Transplant 35(12):e14478
Otani J, Ohta R, Sano C (2021) Association between immunoglobulin G levels and adverse effects following vaccination with the BNT162b2 vaccine among Japanese Healthcare Workers. Vaccines (Basel) 9(10):1149
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
The study was approved by the Institutional Review Board (IRB) of Meir Medical Center, registered under identifier 0056–21. All participants provided written informed consent for participation in the study.
Disclosures
None.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The name of the co-author, Oshrat E. Tayer‑Shifman has been corrected.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pri-Paz Basson, Y., Tayer-Shifman, O.E., Naser, R. et al. Immunogenicity and safety of the mRNA-based BNT162b2 vaccine in systemic autoimmune rheumatic diseases patients. Clin Rheumatol 41, 3879–3885 (2022). https://doi.org/10.1007/s10067-022-06348-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-022-06348-z