Abstract
Objective
Anti-cyclic citrullinated peptide 2 antibodies (anti-CCP2) and rheumatoid factor (RF) in rheumatoid arthritis (RA) has been extensively assessed in industrialized countries. We investigated the diagnostic and prognostic impact of anti-CCP2 and RF isotypes in a Sudanese cross-sectional RA cohort.
Methods
Consecutive RA patients (n = 281) diagnosed according to the 1987 ACR criteria were included 2008–2010. Anti-CCP2 and RF isotypes (IgA, IgM, and IgG) were measured by enzyme immunoassay in 262 patients, with reference intervals aligned to the same diagnostic specificity as for anti-CCP2 (97.6%) using national controls.
Results
IgA RF was the predominant RA-associated autoantibody (56%), followed by IgM RF and anti-CCP2 (both 52%) and IgG RF (49%). In receiver operator characteristic analysis, IgA RF also showed the largest area under the curve. Patients with IgG RF were younger and had 8 years lower median age of disease onset compared to antibody negative patients (p < 0.0001). IgG RF was the only marker associated with a high number of involved joints (p = 0.028), and together with anti-CCP2 were the strongest markers for finger deformities (p = 0.016 and p = 0.012), respectively. No statistical differences were found for disease duration, ESR and Hb levels, and occurrence of erosions/osteopenia for any of the investigated autoantibodies.
Conclusion
Whereas IgA RF showed the best diagnostic performance, IgG RF associated with low age of RA onset, high number of involved joints, and finger deformities. These findings indicate that RA-associated antibodies other than conventional IgM RF and anti-CCP2 might be informative in non-Caucasian RA populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The autoantibody rheumatoid factor (RF) was the first described rheumatoid arthritis (RA)-associated marker and included in the 1987 classification criteria of the American College of Rheumatology (ACR) [1]. After the discovery of anti-cyclic citrullinated protein/peptide antibodies (ACPA), both RF and ACPA were included as serological markers in the new European League Against Rheumatism (EULAR)/ACR classification criteria for RA [2].
Comparison of the diagnostic and prognostic impact of ACPA and RF in RA has been performed more extensively in the industrialized countries than in Africa [3, 4]. In the industrialized countries, the diagnostic utility of the most commonly used ACPA test measuring antibodies against cyclic citrullinated peptide 2 (anti-CCP2) and RF were investigated in systemic reviews performed by Avouac et al. and Nishimura et al. These studies concluded that anti-CCP2 was a better marker for RA diagnosis [5, 6] as well as a better predictor of bone erosions [6] than RF in cohorts mainly encompassing Caucasian RA patients.
What is a positive autoantibody result is not clearly defined, and reference intervals have not been standardized in RA classification. Whereas the 1987 ACR classification criteria state that the reference range should be established so that < 5% of healthy controls are RF positive, thus without imposing any upper limit [1], the 2010 EULAR/ACR criteria give no information about reference ranges, but refer to the “upper limit of normal for the laboratory and assay,” thus leaving establishment of reference interval at the discretion of the individual laboratory [2]. Low levels of autoantibodies are found also in healthy control populations, and to perform proper comparison of performance, the different measures should be aligned to show the same diagnostic specificity in relation to control groups. It is also a common perception among clinical pathologists that levels of clinical laboratory measures differ between populations [7,8,9], and therefore, autoantibody reference intervals should preferably be set in relation to geographically matched control groups.
We recently published a paper characterizing a Sudanese cross-sectional RA cohort and comparing disease activity, treatment, and occurrence of IgM RF among Sudanese and Swedish RA patients [10]. Nothing has been published to date concerning the diagnostic and prognostic properties of anti-CCP2 and RF isotypes in Sudanese RA patients. To address this issue, we undertook a hospital-based study in an RA cohort collected at two major rheumatology outpatient clinics in Khartoum, and compared anti-CCP2, IgA, IgM, and IgG RF concerning diagnostic performance and association to clinical variables among Sudanese RA patients, after aligning all reference ranges to the same diagnostic specificity compared to Sudanese controls.
Methods
Patients and control subjects
This cross-sectional hospital-based study was performed in two rheumatological outpatient units in Khartoum (Alribat University Hospital and Omdurman Military Hospital, Khartoum). Blood samples and patient’s clinical records were collected between December 2008 and September 2010. Newly diagnosed RA patients were included consecutively; only about 2% of the patients attending the hospital did not want to participate [10]. All patients had been diagnosed by rheumatology specialists (MIEA, EME, MAMN) according to the 1987 ACR classification criteria [1] and were included at their first regular follow-up visit during the inclusion period. A total of 281 consecutive Sudanese RA patients were included; 89.3% (251/281) were females. As controls, 180 healthy blood donors (median age 35 years, 89% (161/180) males) from the blood banks of Alribat University Hospital and Soba Teaching Hospital were recruited as described [10]. The procedures followed were in accordance with the Declaration of Helsinki. Ethical approval was obtained from the Ethical Committee of Alribat University Hospital and Omdurman Military Hospital prior to the study, and informed consent was obtained from all patients and controls before sampling. Ethical clearance for performing autoantibody analyses in Uppsala was obtained from the regional ethical board in Uppsala.
The clinical data included age, sex, disease duration, and the number of tender joints according to the EULAR 28 joint count [11]. Data for erythrocyte sedimentation rate (ESR), blood hemoglobin (Hb) level, and X-rays of the hands including data of the occurrence of erosions and osteopenia was obtained from the patient records for 169, 176, and 60 of the patients, respectively. Information about hand and wrist deformities (Z deformity (ZD), swan neck deformity (SND), boutonniere deformity (BD), and ulnar deviation (UD)) was recorded for 252 RA patients at the time of study inclusion. Age at disease onset was calculated by subtracting disease duration from age at study inclusion. Only three of 255 patients who responded to the question about smoking had ever smoked. Details about the retrieval of data and characterization of the Sudanese RA patients have been published [10].
Autoantibody measurements
IgA, IgG, and IgM RF isotypes and anti-CCP2 of the conventionally measured IgG isotype were investigated using an enzyme immune assay (Elia, Phadia Thermo Fisher, Uppsala, Sweden) according to the manufacturer’s instructions. Anti-CCP2 was considered positive when the concentration was > 7 arbitrary units (AU)/ml, in accordance with the reference range utilized at Uppsala University Hospital, and representing the lower limit of the cutoff range suggested by the manufacturer. Using this reference interval, 4/168 Sudanese controls were anti-CCP2 positive, corresponding to a diagnostic specificity of 97.6%. We then applied the same specificity level for the RF isotypes in relation to the same control group, and in accordance with the definition in the ACR criteria (< 5% positive individual in a healthy reference group [1]). One healthy 29-year-old male co-expressed IgM RF and IgA RF. Otherwise, autoantibodies occurred isolated among the controls; the other single positive controls followed the age distribution of that group. Measurement ranges for anti-CCP2 were 0.4–≥ 340 AU/ml, and for IgA, IgM, and IgG RF 0.4–≥ 214 international units (IU)/ml, 0.4–200 IU/ml, and up to 600 μg/ml, respectively. For statistical reasons, values above the reference range were noted as 400 AU/ml, 250 IU/ml, and 250 IU/ml for anti-CCP2, IgA, and IgM, respectively. All values for IgG RF were within the measurement range. Data on IgA, IgG, and IgM RF and anti-CCP2 were obtained for 248, 253, 250, and 262 patients, respectively. Full data on all four autoantibodies were available in 240 patients.
Statistics
As the distribution of RA-associated autoantibodies, especially ACPA, is non-normal, non-parametric tests were used. The Mann-Whitney was used to test for differences between groups, and the χ2 test was used to test for differences between proportions. Two-way ANOVA was used to evaluate any interaction between anti-CCP2 and IgG RF as independent variables on age of RA onset as independent variable. The effects of four autoantibodies as independent variables were evaluated with age of RA onset and number of hand deformities as independent variables in multiple regression. Here occurrence of individual autoantibodies were used as nominal variables as the distribution of anti-CCP2 and IgM RF was bimodal with many patients with levels above the measurement range. P values < 0.05 were considered significant. Analyses were performed using the JMP software (SAS institute, Cary, NC, USA). Receiver operator characteristics (ROC) curves were constructed and area under the curve (AUC) was measured using the Analyze-it software (Leeds, UK).
Results
Diagnostic impact of RA-associated autoantibodies
Using the uniform diagnostic specificity alignment described above, we obtained the cutoffs > 9.1 IU/ml, > 3.9 IU/ml, and > 35 μg/ml for IgA, IgM, and IgG RF, respectively, and subsequently used in this study.
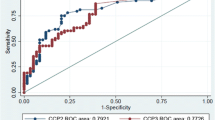
Anti-CCP2 levels were elevated in 52% (137/262) of the RA patients. IgA RF was positive in 56% (139/248), IgG RF in 49% (124/253), and IgM RF in 52% (131/250) of the investigated patients. The AUC were 0.81 for anti-CCP2, 0.85 for IgA, 0.81 for IgG, and 0.76 for IgM RF (Fig. 1a and Table 1). The AUC was significantly larger for IgA RF than for IgM RF (p = 0.001) and IgG RF (p = 0.042), but was not different than for anti-CCP2 (p = 0.11; Table 1).
a Receiver operator characteristics (ROC) curve comparing the sensitivity and specifity for anti-CCP2, IgA RF, IgM RF, and IgG RF. Curves are based on 248, 253, 250, and 262 patients for IgA, IgG, and IgM RF, and for anti-CCP2, respectively, and compared to 180 healthy controls. b Venn diagram showing the co-occurrence of IgG anti-CCP2, IgA RF, IgG RF, and IgM RF among the 240 RA patients with complete autoantibody data. Fiftynine patients did not have any autoantibody reactivity
Levels of all investigated autoantibodies correlated significantly with one another. The strongest correlation was between anti-CCP2 and IgA RF (Spearman’s ρ = 0.64). IgG RF generally showed the lowest level of correlation to other autoantibodies. The degree of co-occurrence of all four autoantibodies are shown as a Venn diagram in Fig. 1b for the 240 patients with complete data.
Prognostic impact of RA-associated autoantibodies
The age at inclusion did not differ between patients with and without IgA RF and IgM RF but was significantly lower among anti-CCP2 and IgG RF positive as compared to patients without the corresponding autoantibody (median 48 vs. 50 years, p = 0.019 and median 47 vs. 51 years, p = 0.003; Fig. 2 and Table 2). Patients with anti-CCP2, IgA RF, IgG RF, and IgM RF were significantly younger at disease onset compared to antibody negative patients (p = 0.003, p = 0.014, < 0.0001, and p = 0.029, respectively); for IgG RF, the difference was 8 years (40 vs. 48 years; Fig. 3 and Table 2).
As onset age was strongly dependent both on anti-CCP2 and on IgG RF, we stratified patients according to anti-CCP2 and IgG RF, respectively. The median age of onset was statistically lower in IgG RF-positive patients when compared to IgG RF-negative patients both among anti-CCP2-positive and anti-CCP2-negative patients (p = 0.041 and p = 0.002, respectively; Table 3). On the contrary, no difference in age of onset was found between anti-CCP2-positive and -negative patients dichotomized according to IgG RF status (Table 3). Two-way ANOVA analysis yielded similar results, as age at RA onset associated with IgG RF but not with anti-CCP2 (p = 0.0005 and p = 0.191, respectively), without any statistical interaction between the antibodies (data not shown). Multiple regression using occurrence of anti-CCP2, IgA RF, IgG RF, and IgM RF as independent variables again showed that only IgG RF was significantly associated with low age of onset (p = 0.0017, standardized β 0.23).
The proportion of female patients with anti-CCP2 or IgA RF was significantly higher than the proportion of females without anti-CCP2 or IgA RF, respectively. No corresponding difference was evident for IgG and IgM RF (Table 2). ESR, Hb, WBC, occurrence of erosions/osteopenia, and number of affected joints were not different among patients with or without any autoantibody (Table 2).
Individual finger and hand deformities associated weakly with occurrence of different autoantibodies, but only IgG RF showed strong associations with the occurrence of SND (p = 0.0003) and BD (p = 0.009; Table 2). In multiple regression using occurrence of anti-CCP2, IgA RF, IgG RF, and IgM RF as independent variables, only IgG RF was significantly associated with total number of hand deformities (ZD, SND, BD, UD; p = 0.0470, standardized β − 0.15).
Discussion
This is the first report that describes the diagnostic and prognostic properties for RA-associated autoantibodies in a Sudanese RA cohort. We found that IgA RF was the diagnostically most sensitive autoantibody followed by anti-CCP2 and IgM RF and then by IgG RF (49.8%), after that reference intervals had been adjusted to the same diagnostic specificity. The occurrence of anti-CCP2 was rather similar to what has been reported among Swedish RA patients (56%) [12]. A smaller study on 56 Cameroon RA patients by Singwe-Ngandeu et al. showed a higher prevalence of IgA RF (84%) followed by IgM RF (77%), although this was not commented upon in that paper [4]. The frequency order for the different RF isotypes that we report differs from the IgM RF predominance that is commonly described in Caucasian RA patients. A study from Germany by Vallbracht et al. showed that IgM RF (66%) was the predominant type, closely followed by anti-CCP2 (64%), with lower frequencies of IgA RF (51%) and IgG RF (44%) [13]. Another study from Sweden reported the same frequency order for RF isotypes, where IgM RF was detected in 79% of the RA cases, followed by IgA (78%) and IgG RF (68%) [14]. Conversely, Asian RA studies report the lowest frequency for IgA RF. A study from India by Singh et al. showed a relative increase in impact for IgG RF, with 48% IgM RF-positive RA patients, followed by IgG RF (42%) and IgA RF (37%) [15]. A study on 147 Malaysian RA patients [16] reported the same pattern of distribution for RF isotypes as in India [15], IgM RF (53.1%) being the most common autoantibodies in these three different ethnic groups, followed by IgG RF (48.3%) and then by IgA RF (21.1%). In a larger Malaysian study encompassing 171 RA patients in the primary cohort and 886 in the replication cohort, IgG RF sensitivity was comparable to anti-CCP2, but predominated over IgM RF among RA patients of all three ethnicities living in Malaysia: Chinese, Indian, and Malay ancestry [17].
Thus, there seems to a be a relative difference in RF isotype distribution between RA patients from three continents, with IgA RF predominating in Africa [[4] and this study], IgM RF predominating in Europe [13, 14] and with a relative increase in IgG RF and decrease in IgA RF in Asia [15,16,17].
This notion that RA populations from different parts of the world show divergent distribution of RF isotypes raises the question concerning whether this variation is primarily driven by genetic or environmental factors, and we argue for the latter possibility. A report on the predominance of IgM RF (70%) over IgA RF (65%) among African American RA patients [18] corresponds to the isotype distribution among North American native RA patients [19] but differs from black patient in Africa as shown by us. This indicates that the RF isotype pattern is primarily driven by environmental and not by genetic factors, as does the fact that the preponderance of IgG RF is evident in all three ethnically distinct populations in Malaysia [16, 17]. Two studies have reported a predominance of IgA anti-cardiolipin antibodies over the IgG and IgM isotypes in African patients with SLE [20, 21]. The tendency to produce IgA autoantibodies in Africa might therefore not be restricted to RF but include also other autoantibodies and might also be generalized to other humoral immune reactions.
For further comparisons of the diagnostic value of each assay we undertook ROC curve analyses and calculated the AUC. The AUC was highest for IgA RF and statistically different from IgG and IgM RF but not from anti-CCP2. These facts strengthen our perception that IgA RF is the diagnostically most sensitive laboratory marker for detecting RA patients in central Africa, irrespective of what reference ranges are applied. Collectively, our findings suggest that IgA RF may have advantages of other RA-associated autoantibodies as a diagnostic test for RA in central Africa.
A weakness in our study is the sex bias in our control group consisting of mainly male blood donors (very few women donate blood in Sudan) with lower men age compared to the patients. However, probably even more important is that although if IgA RF is superior to anti-CCP2 when the reference interval is set according to national healthy controls, what really matters in real life health care is how the antibodies perform compared to disease controls with the differential diagnoses seen in Sudan. The clinical breakthrough for anti-CCP2 came when this antibody proved to have superior specificity compared to RF compared to disease controls with rheumatic conditions other than RA and infections, respectively [22]. It is also possible that RF isotype patterns change over time, and that the isotype distribution in this cross-sectional cohort with a median of 3 years of disease duration differs from newly diagnosed patients. To really prove our hypothesis that IgA RF is the superior diagnostic marker, an incident RA cohort should be investigated, and reference intervals established in relation to national relevant disease controls.
Of the investigated autoantibodies, IgG RF was most strongly associated with severe disease due to its association with younger age at diagnosis, conspicuously lower age of disease onset, and high number of involved joint deformities. Anti-CCP2 was also found to be associated with severe disease as commonly described among Caucasian RA patients [23, 24]. Previous studies have indicated associations between bone destruction and RF isotypes [25,26,27], but we could not repeat these findings. The quality of our radiology data is however limited and based on qualitative evaluation of X-ray data performed by different radiologists at different time points in 21% of the patient cohort.
Intriguingly, when we looked for a correlation between anti-CCP2 and the different RF isotypes in our Sudanese RA cohort, the weakest correlation was found between IgG RF and anti-CCP2. This indicates that the clinical associations between IgG RF and early RA onset/extensive joint involvement found in our cohort are not a result of co-variation with anti-CCP2, but truly associated to IgG RF. When we stratified patients according to anti-CCP2 and IgG RF, we found that low age of RA onset associated with IgG RF, but not with anti-CCP2; this was also corroborated by two-way ANOVA. Our findings therefore suggest that the IgG RF is the strongest autoantibody marker for early RA onset among RA patients in Sudan. A previous Danish study reported that IgM RF as in this study was weakly associated with young age at RA onset (p = 0.03 and p = 0.029, respectively), but did not include any data on other RF isotypes [28]. Again, these findings re-emphasize the importance of discrete evaluation of individual RF isotypes in RA populations from different parts of the world.
Conclusions
IgA seems to be the diagnostically most sensitive autoantibody marker for RA in Sudan, although crucial comparisons with national disease controls in incident RA cohorts are currently lacking. IgG RF is the marker most strongly associated with young age of disease onset and with the occurrence of classical hand deformities. Thus, although IgM RF is mostly investigated for the classification and diagnosis of RA in Caucasian populations, other RF isotypes might be more informative in other ethnic groups.
References
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS et al (1988) The American rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31(3):315–324
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, Combe B, Costenbader KH, Dougados M, Emery P, Ferraccioli G, Hazes JM, Hobbs K, Huizinga TW, Kavanaugh A, Kay J, Kvien TK, Laing T, Mease P, Menard HA, Moreland LW, Naden RL, Pincus T, Smolen JS, Stanislawska-Biernat E, Symmons D, Tak PP, Upchurch KS, Vencovsky J, Wolfe F, Hawker G (2010) 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 62(9):2569–2581. https://doi.org/10.1002/art.27584
Abdel-Nasser AM, Mahmoud MH, El Mansoury TM, Osman AM (2008) Anti-CCP2 is an adjunct to, not a surrogate for, rheumatoid factor in the diagnosis of rheumatoid arthritis: diagnostic utility of anti-CCP2 antibodies in Egyptian patients with rheumatoid arthritis. Scand J Rheumatol 37(5):329–336. https://doi.org/10.1080/03009740802116208
Singwe-Ngandeu M, Finckh A, Bas S, Tiercy JM, Gabay C (2010) Diagnostic value of anti-cyclic citrullinated peptides and association with HLA-DRB1 shared epitope alleles in African rheumatoid arthritis patients. Arthritis Res Ther 12(2):R36. https://doi.org/10.1186/ar2945
Avouac J, Gossec L, Dougados M (2006) Diagnostic and predictive value of anti-cyclic citrullinated protein antibodies in rheumatoid arthritis: a systematic literature review. Ann Rheum Dis 65(7):845–851. https://doi.org/10.1136/ard.2006.051391
Nishimura K, Sugiyama D, Kogata Y, Tsuji G, Nakazawa T, Kawano S, Saigo K, Morinobu A, Koshiba M, Kuntz KM, Kamae I, Kumagai S (2007) Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Ann Intern Med 146(11):797–808
Fida S, Myers M, Mackay IR, Zimmet PZ, Mohan V, Deepa R, Rowley MJ (2001) Antibodies to diabetes-associated autoantigens in Indian patients with type 1 diabetes: prevalence of anti-ICA512/IA2 and anti-SOX13. Diabetes Res Clin Pract 52(3):205–211
Korpilahde T, Heliovaara M, Kaipiainen-Seppanen O, Knekt P, Aho K (2003) Regional differences in Finland in the prevalence of rheumatoid factor in the presence and absence of arthritis. Ann Rheum Dis 62(4):353–355
Shapira Y, Poratkatz BS, Gilburd B, Barzilai O, Ram M, Blank M, Lindeberg S, Frostegard J, Anaya JM, Bizzaro N, Jara LJ, Damoiseaux J, Shoenfeld Y, Levin NA (2012) Geographical differences in autoantibodies and anti-infectious agents antibodies among healthy adults. Clin Rev Allergy Immunol 42(2):154–163. https://doi.org/10.1007/s12016-010-8241-z
Elshafie AI, Elkhalifa AD, Elbagir S, Aledrissy MI, Elagib EM, Nur MA, Weitoft T, Ronnelid J (2016) Active rheumatoid arthritis in Central Africa: a comparative study between Sudan and Sweden. J Rheumatol 43(10):1777–1786. https://doi.org/10.3899/jrheum.160303
Prevoo ML, van ‘t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL (1995) Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 38(1):44–48
Rönnelid J, Wick MC, Lampa J, Lindblad S, Nordmark B, Klareskog L, van Vollenhoven RF (2005) Longitudinal analysis of citrullinated protein/peptide antibodies (anti-CP) during 5 year follow up in early rheumatoid arthritis: anti-CP status predicts worse disease activity and greater radiological progression. Ann Rheum Dis 64(12):1744–1749. https://doi.org/10.1136/ard.2004.033571
Vallbracht I, Rieber J, Oppermann M, Forger F, Siebert U, Helmke K (2004) Diagnostic and clinical value of anti-cyclic citrullinated peptide antibodies compared with rheumatoid factor isotypes in rheumatoid arthritis. Ann Rheum Dis 63(9):1079–1084. https://doi.org/10.1136/ard.2003.019877
Lindqvist E, Eberhardt K, Bendtzen K, Heinegard D, Saxne T (2005) Prognostic laboratory markers of joint damage in rheumatoid arthritis. Ann Rheum Dis 64(2):196–201. https://doi.org/10.1136/ard.2003.019992
Singh U, Vishwanath A, Verma PK, Singh NK, Shukla RC, Singh S, Singh S, Sonkar GK (2010) Is rheumatoid factor still a superior test for the diagnosis of rheumatoid arthritis? Rheumatol Int 30(8):1115–1119. https://doi.org/10.1007/s00296-009-1338-0
Gomez EL, Gun SC, Somnath SD, D'Souza B, Lim AL, Chinna K, Radhakrishnan AK (2011) The prevalence of rheumatoid factor isotypes and anti-cyclic citrullinated peptides in Malaysian rheumatoid arthritis patients. Int J Rheum Dis 14(1):12–17. https://doi.org/10.1111/j.1756-185X.2010.01573.x
Too CL, Rönnelid J, Mat Yusoff Y, Singh Dhaliwal J, Ashikin Jinah N, Yahya A, Hussien H, Sulaiman W, Larsson PT, Murad S (2014) Increased IgG rheumatoid factor-positivity in the Asian rheumatoid arthritis patients irrespective of ethnicity. Open J Rheumatol Autoimmune Dis 4:43–51
Mikuls TR, Holers VM, Parrish L, Kuhn KA, Conn DL, Gilkeson G, Smith EA, Kamen DL, Jonas BL, Callahan LF, Alarcon GS, Howard G, Moreland LW, Bridges SL Jr (2006) Anti-cyclic citrullinated peptide antibody and rheumatoid factor isotypes in African Americans with early rheumatoid arthritis. Arthritis Rheum 54(9):3057–3059. https://doi.org/10.1002/art.22200
Hitchon CA, Chandad F, Ferucci ED, Willemze A, Ioan-Facsinay A, van der Woude D, Markland J, Robinson D, Elias B, Newkirk M, Toes RM, Huizinga TW, El-Gabalawy HS (2010) Antibodies to porphyromonas gingivalis are associated with anticitrullinated protein antibodies in patients with rheumatoid arthritis and their relatives. J Rheumatol 37(6):1105–1112. https://doi.org/10.3899/jrheum.091323
Cucurull E, Gharavi AE, Diri E, Mendez E, Kapoor D, Espinoza LR (1999) IgA anticardiolipin and anti-beta2-glycoprotein I are the most prevalent isotypes in African American patients with systemic lupus erythematosus. Am J Med Sci 318(1):55–60
Gould T, Tikly M, Asherson R, Loizou S, Singh S (2006) Prevalence and clinical correlates of anti-phospholipid antibodies in south Africans with systemic lupus erythematosus. Scand J Rheumatol 35(1):29–34. https://doi.org/10.1080/03009740510026913
Schellekens GA, Visser H, de Jong BA, van den Hoogen FH, Hazes JM, Breedveld FC, van Venrooij WJ (2000) The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum 43(1):155–163. https://doi.org/10.1002/1529-0131(200001)43:1<155::AID-ANR20>3.0.CO;2-3
Machold KP, Stamm TA, Nell VP, Pflugbeil S, Aletaha D, Steiner G, Uffmann M, Smolen JS (2007) Very recent onset rheumatoid arthritis: clinical and serological patient characteristics associated with radiographic progression over the first years of disease. Rheumatology (Oxford) 46(2):342–349. https://doi.org/10.1093/rheumatology/kel237
Willemze A, Toes RE, Huizinga TW, Trouw LA (2012) New biomarkers in rheumatoid arthritis. Neth J Med 70(9):392–399
Ahmed MM, Obaid Al-Ruhaimi KA, Mohammed SH (2010) Evaluation of the rheumatoid factors of the IgG, IgM and IgA isotypes as prognostic parameters for rheumatoid arthritis among Iraqi patients. Indian J Pathol Microbiol 53(3):433–438. https://doi.org/10.4103/0377-4929.68265
van Leeuwen MA, Westra J, van Riel PL, Limburg PC, van Rijswijk MH (1995) IgM, IgA, and IgG rheumatoid factors in early rheumatoid arthritis predictive of radiological progression? Scand J Rheumatol 24(3):146–153
Winska Wiloch H, Thompson K, Young A, Corbett M, Shipley M, Hay F (1988) IgA and IgM rheumatoid factors as markers of later erosive changes in rheumatoid arthritis (RA). Scand J Rheumatol Suppl 75:238–243
Jacobsen S (2004) Young age of onset is associated with increased prevalence of circulating IgM rheumatoid factor and antinuclear antibodies at presentation in women with rheumatoid arthritis. Clin Rheumatol 23(2):121–122. https://doi.org/10.1007/s10067-003-0844-9
Acknowledgments
The company Phadia/Thermo Fischer Scientific provided reagents for analysis of RF isotypes and anti-CCP2.
Funding
Financial support was obtained from the Swedish Research Council (grant number K2014-68X-20611-07-3 to J.R.), the Swedish Rheumatism Association, Agnes and Mac Rudberg Foundation, King Gustav Vth 80-Year Foundation, and the Signe and Reinhold Sund’s Foundation for Rheumatological Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Ethical standards
The study has been approved by appropriate ethics committees, and the research was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. All subjects gave their informed consent prior to inclusion in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Elshafie, A.I., Elbagir, S., Aledrissy, M.I.E. et al. Occurrence of anti-CCP2 and RF isotypes and their relation to age and disease severity among Sudanese patients with rheumatoid arthritis. Clin Rheumatol 38, 1545–1553 (2019). https://doi.org/10.1007/s10067-019-04431-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-019-04431-6