Abstract
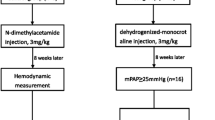
Urotensin II (UII) has been reported to play a key role in pulmonary arterial hypertension (PAH) development. Doppler echocardiography, a noninvasive and simple tool, is recommended for diagnosing PAH. This study was designed to investigate the effect of urantide, a UII receptor antagonist, on the structure and function of the right ventricle in PAH rat models by Doppler echocardiography. A total of 60 male rats were divided into two groups: early- and late-treatment groups. Rats in the urantide and MCT (monocrotaline) subgroups were injected with 10 μg/kg urantide in the urantide group or an equal amount of normal saline in the MCT group 1 week after PAH model construction in the early-treatment group and 4 weeks after the construction in the late-treatment group. Rats in the control group received an equal volume of normal saline solution. PAH-related indexes were measured by echocardiography. PAH rat models exhibited higher right ventricular diastolic diameter and lower time to peak, ejection time, and peak flow velocity of pulmonary artery than controls (P < 0.05). However, compared with the MCT group, all abovementioned indexes were improved in the urantide group (P < 0.05). No significant differences in pulmonary artery diameter and left ventricular ejection fraction were noted among the groups. Compared with the MCT group, systolic pulmonary arterial pressure (SPAP) and mean pulmonary arterial pressure (mPAP) were significantly lower in the urantide group (P < 0.05). SPAP examined by echocardiography was correlated with mPAP by catheterization (P < 0.05). Urantide treatment improved right heart failure parameters in MCT-induced PAH rats, thus providing a potential new strategy for treating PAH.



Similar content being viewed by others
References
Barst RJ, Mcgoon M, Torbicki A, Sitbon O, Krowka MJ, Olschewski H, Gaine S (2004) Diagnosis and differential assessment of pulmonary arterial hypertension. J Am Coll Cardiol 43(12):S40–S47. https://doi.org/10.1016/j.jacc.2004.02.032
MH X, Gong YS, MS S, Dai ZY, Dai SS, Bao SZ, Li N, Zheng RY, He JC, Chen JF (2010) Absence of the adenosine A2A receptor confers pulmonary arterial hypertension and increased pulmonary vascular remodeling in mice. J Vasc Res 48(2):171–183
Essop MR (2010) Contemporary insights into the pathogenesis, diagnosis and therapy of pulmonary arterial hypertension. Cardiovasc J Afr 21(6):334–337. https://doi.org/10.5830/CVJA-2010-088
Proulx CD, Holleran BJ, Lavigne P, Escher E, Guillemette G, Leduc R (2008) Biological properties and functional determinants of the urotensin II receptor. Peptides 29(5):691–699. https://doi.org/10.1016/j.peptides.2007.10.027
Bottrill FE, Douglas SA, Hiley CR, White R (2000) Human urotensin-II is an endothelium-dependent vasodilator in rat small arteries. Br J Pharmacol 130(8):1865–1870. https://doi.org/10.1038/sj.bjp.0703513
Sauzeau V, Le ME, Bertoglio J, Scalbert E, Pacaud P, Loirand G (2001) Human urotensin II-induced contraction and arterial smooth muscle cell proliferation are mediated by RhoA and Rho-kinase. Circ Res 88(11):1102–1104. https://doi.org/10.1161/hh1101.092034
Takahashi K, Totsune K, Murakami O, Arihara Z, Noshiro T, Hayashi Y, Shibahara S (2003) Expression of urotensin II and its receptor in adrenal tumors and stimulation of proliferation of cultured tumor cells by urotensin II. Peptides 24(2):301–306. https://doi.org/10.1016/S0196-9781(03)00039-1
Coulouarn Y, Lihrmann I, Jegou S, Anouar Y, Tostivint H, Beauvillain JC, Conlon JM, Bern HA, Vaudry H (1998) Cloning of the cDNA encoding the urotensin II precursor in frog and human reveals intense expression of the urotensin II gene in motoneurons of the spinal cord. Proc Natl Acad Sci U S A 95(26):15803–15808. https://doi.org/10.1073/pnas.95.26.15803
Onan D, Hannan RD, Thomas WG (2004) Urotensin II: the old kid in town. Trends Endocrinol Metab Tem 15(4):175–182. https://doi.org/10.1016/j.tem.2004.03.007
Chatenet D, Dubessy C, Leprince J, Boularan C, Carlier L, Ségalas-Milazzo I, Guilhaudis L, Oulyadi H, Davoust D, Scalbert E (2004) Structure-activity relationships and structural conformation of a novel urotensin II-related peptide. Peptides 25(10):1819–1830. https://doi.org/10.1016/j.peptides.2004.04.019
Simpson CM, Smolich JJ, Shekerdemian LS, Penny DJ (2010) Urotensin-II contributes to pulmonary vasoconstriction in a perinatal model of persistent pulmonary hypertension of the newborn secondary to meconium aspiration syndrome. Pediatr Res 67(2):150–157. https://doi.org/10.1203/PDR.0b013e3181c345ea
Gao S, Oh YB, Shah A, Park WH, Chung MJ, Lee YH, Kim SH (2010) Urotensin II receptor antagonist attenuates monocrotaline-induced cardiac hypertrophy in rats. Am J Physiol Heart Circ Physiol 299(6):H1782–H1789. https://doi.org/10.1152/ajpheart.00438.2010
Mei Y, Jin H, Tian W, Wang H, Wang H, Zhao Y, Zhang Z, Meng F (2011) Urantide alleviates monocrotaline induced pulmonary arterial hypertension in Wistar rats. Pulm Pharmacol Ther 24(4):386–393. https://doi.org/10.1016/j.pupt.2011.03.003
Rubin LJ (1993) Primary pulmonary hypertension. Chest 104(1):236–250. https://doi.org/10.1378/chest.104.1.236
Rich S, Shillington A, Mclaughlin V (2003) Comparison of survival in patients with pulmonary hypertension associated with fenfluramine to patients with primary pulmonary hypertension. Am J Cardiol 92(11):1366–1368. https://doi.org/10.1016/j.amjcard.2003.08.034
Mclaughlin VV, Presberg KW, Doyle RL, Abman SH, Mccrory DC, Fortin T, Ahearn G (2004) Prognosis of pulmonary arterial hypertension*: ACCP evidence-based clinical practice guidelines. Chest 126(1 Suppl):78S–92S. https://doi.org/10.1378/chest.126.1_suppl.78S
Safdar Z (2011) Treatment of pulmonary arterial hypertension: the role of prostacyclin and prostaglandin analogs. Respir Med 105(6):818–827. https://doi.org/10.1016/j.rmed.2010.12.018
Ghofrani HA, Pepke-Zaba J, Barbera JA, Channick R, Keogh AM, Gomez-Sanchez MA, Kneussl M, Grimminger F (2004) Nitric oxide pathway and phosphodiesterase inhibitors in pulmonary arterial hypertension. J Am Coll Cardiol 43(12):S68–S72. https://doi.org/10.1016/j.jacc.2004.02.031
Galié N, Manes A, Branzi A (2004) The endothelin system in pulmonary arterial hypertension. Cardiovasc Res 61(2):227–237. https://doi.org/10.1016/j.cardiores.2003.11.026
Galiè N, Humbert M, Vachiery J-L, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Noordegraaf AV, Beghetti M (2016) 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 31(1):1219–1263
Stenmark KR, Meyrick B, Galie N, Mooi WJ, McMurtry IF (2009) Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell Mol Physiol 297(6):L1013–L1032. https://doi.org/10.1152/ajplung.00217.2009
Tran L, Kompa AR, Phrommintikul A, Wang BH, Krum H (2010) Chronic urotensin-II infusion induces diastolic dysfunction and enhances collagen production in rats. Ajp Heart Circ Physiol 298(298):H608–H613. https://doi.org/10.1152/ajpheart.00942.2009
Liuhua S (2010) Analysis of right ventricular function in patients with pulmonary hypertension using volume-time curves by real-time three-dimensinal echocardiography. Journal of Clin Cardiol 26(12):898–901
Mukerjee D, St GD, Knight C, Davar J, Wells AU, BR D, Black CM, Coghlan JG (2004) Echocardiography and pulmonary function as screening tests for pulmonary arterial hypertension in systemic sclerosis. Rheumatology 43(4):461–466. https://doi.org/10.1093/rheumatology/keh067
Bossone E, Bodini BD, Mazza A, Allegra L (2005) Pulmonary arterial hypertension: the key role of echocardiography. Chest 127(5):1836–1843. https://doi.org/10.1378/chest.127.5.1836
Condliffe R, Radon M, Hurdman J, Davies C, Hill C, Akil M, Guarasci F, Rajaram S, Swift AJ, Wragg Z, van Beek E, Elliot CA, Kiely DG (2011) CT pulmonary angiography combined with echocardiography in suspected systemic sclerosis-associated pulmonary arterial hypertension. Rheumatology (Oxf, Engl) 50(8):1480–1486. https://doi.org/10.1093/rheumatology/ker114
Berger M, Haimowitz A, Van Tosh A, Berdoff RL, Goldberg E (1985) Quantitative assessment of pulmonary hypertension in patients with tricuspid regurgitation using continuous wave Doppler ultrasound. J Am Coll Cardiol 6(2):359–365. https://doi.org/10.1016/S0735-1097(85)80172-8
Kosturakis D, Goldberg SJ, Allen HD, Loeber C (1984) Doppler echocardiographic prediction of pulmonary arterial hypertension in congenital heart disease. Am J Cardiol 53(8):1110–1115. https://doi.org/10.1016/0002-9149(84)90646-5
Villamor E, Kessels CG, Ruijtenbeek K, Van Suylen RJ, Belik J, De Mey JG, Blanco CE (2004) Chronic in ovo hypoxia decreases pulmonary arterial contractile reactivity and induces biventricular cardiac enlargement in the chicken embryo. Am J Phys Regul Integr Comp Phys 287(3):R642–R651
Lijnen P, Petrov V, Fagard R (2000) Induction of cardiac fibrosis by transforming growth factor-β 1. Mol Genet Metab 71(1):418–435. https://doi.org/10.1006/mgme.2000.3032
Funding
This work was supported by the Science and Technology Research Project of the Education Department of Heilongjiang Province (Program No. 12531239).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experiments were approved by the Ethics Committee of Experimental Research in the First Affiliated Hospital of Harbin Medical University and conformed to the Guide for the Care and Use of Laboratory Animals.
Disclosures
None.
Additional information
Highlights
A monocrotaline-induced PAH rat model was established.
Urantide benefited the structure and function of the right ventricle in PAH rats.
Urantide improved the hemodynamic parameters of PAH rats.
SPAP examined by echocardiography was significantly correlated with mPAP value.
Electronic supplementary material
ESM 1
(DOCX 45 kb)
Rights and permissions
About this article
Cite this article
Wang, Y., Tian, W., Xiu, C. et al. Urantide improves the structure and function of right ventricle as determined by echocardiography in monocrotaline-induced pulmonary hypertension rat model. Clin Rheumatol 38, 29–35 (2019). https://doi.org/10.1007/s10067-018-3978-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-018-3978-5