Abstract
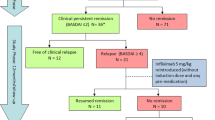
The aim of this study is to assess the recurrence probability and the possible predictors in patients with ankylosing spondylitis from etanercept discontinuation in a 3-year observational cohort (ClinicalTrials.gov: NCT02915354). A cohort of 35 patients who achieved an ASAS 20 response at the end of a randomized controlled trial underwent a 3-year follow-up evaluation. The primary end point was clinical relapse defined as the BASDAI score going back to 80% of its initial level at the beginning of the trial. Prognostic factors of relapse were analyzed using the Cox regression. Median duration of clinical remission was 15.0 months (interquartile range, 3.7–26.3 months). The cumulative probabilities of relapse at 1, 2, and 3 years were 45.7, 57.1, and 60.0%, respectively. The proportion of recurrence was not significantly different between placebo group and etanercept group by Kaplan-Meier analysis (placebo vs. etanercept: 61.11 vs. 58.82%, P = 0.890). Two independent factors associated with increasing risk of relapse were (1) age of patients (25 years or older with risk of 3.07, 95% confidence interval, 1.19–7.97, P = 0.021); (2) onset age (younger than 24 years with risk of 3.12, 95% confidence interval, 1.24–7.83, P = 0.016). No correlation was observed in the present study between the time of relapse and the duration of the treatment with etanercept in AS patients who achieved the ASAS 20 response after receiving the treatment. The older age and younger onset age of patients seems to be important factors associate with an increasing risk of relapse.


Similar content being viewed by others
References
Braun J, Sieper J (1996) The sacroiliac joint in the spondyloarthropathies. Curr Opin Rheumatol 8:275–287
de Vlam K, Mielants H, Cuvelier C et al (2000) Spondyloarthropathy is underestimated in inflammatory bowel disease: prevalence and HLA association. J Rheumatol 27:2860–2865
Braun J, de Keyser F, Brandt J et al (2001) New treatment options in spondyloarthropathies: increasing evidence for significant efficacy of anti-tumor necrosis factor therapy. Curr Opin Rheumatol 13:245–249
Gorman JD, Sack KE, Davis JC Jr (2002) Treatment of ankylosing spondylitis by inhibition of tumor necrosis factor alpha. N Engl J Med 346:1349–1356
Baji P, Pentek M, Szanto S et al (2014) Comparative efficacy and safety of biosimilar infliximab and other biological treatments in ankylosing spondylitis: systematic literature review and meta-analysis. Eur J Health Econ 15(Suppl 1):S45–S52
Li ZH, Zhang Y, Wang J et al (2013) Etanercept in the treatment of ankylosing spondylitis: a meta-analysis of randomized, double-blind, placebo-controlled clinical trials, and the comparison of the Caucasian and Chinese population. Eur J Orthop Surg Traumatol 23:497–506
Kiltz U, Baraliakos X, Fau Braun J, van der Heijde D et al (2013) Withdrawal of medical therapies in axial spondyloarthritis: what would be the optimal trial design? Clin Exp Rheumatol 31:S47–S50
Baraliakos X, Listing J, Brandt J et al (2005) Clinical response to discontinuation of anti-TNF therapy in patients with ankylosing spondylitis after 3 years of continuous treatment with infliximab. Arthritis Res Ther 7:R439–R444
Baraliakos X, Listing J, Rudwaleit M et al (2007) Safety and efficacy of readministration of infliximab after longterm continuous therapy and withdrawal in patients with ankylosing spondylitis. J Rheumatol 34:510–515
Brandt J, Listing J, Haibel H et al (2005) Long-term efficacy and safety of etanercept after readministration in patients with active ankylosing spondylitis. Rheumatology (Oxford) 44:342–348
Lin Q, Lin ZF, Gu J, Gu JF, Huang F et al (2010) Abnormal high-expression of CD154 on T lymphocytes of ankylosing spondylitis patients is down-regulated by etanercept treatment. Rheumatol Int 30:317–323
Calin A, Garrett S, Whitelock H et al (1994) A new approach to defining functional ability in ankylosing spondylitis: the development of the bath ankylosing spondylitis functional index. J Rheumatol 21:2281–2285
van der Heijde D, Dougados M, Davis J et al (2005) Assessment in Ankylosing Spondylitis International Working Group/Spondylitis Association of America recommendations for conducting clinical trials in ankylosing spondylitis. Arthritis Rheum 52:386–394
Garrett S, Jenkinson T, Kennedy LG et al (1994) A new approach to defining disease status in ankylosing spondylitis: the Bath ankylosing spondylitis disease activity index. J Rheumatol 21:2286–2291
Brandt JF, Haibel H, Haibel HF, Sieper J, Sieper JF, Reddig J et al (2001) Infliximab treatment of severe ankylosing spondylitis: one-year followup. Arthritis Rheum 44:2936–2937
Lukas C, Landewe R, Sieper J et al (2009) Development of an ASAS-endorsed disease activity score (ASDAS) in patients with ankylosing spondylitis. Ann Rheum Dis 68:18–24
van der Heijde D, Lie E, Kvien TK et al (2009) ASDAS, a highly discriminatory ASAS-endorsed disease activity score in patients with ankylosing spondylitis. Ann Rheum Dis 68:1811–1818
Peto RF, Pike MC, Pike MF, Armitage P, Armitage PF, Breslow NE et al (1977) Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II: analysis and examples. Br J Cancer 35:1–39
Braun J, Brandt J, Listing J et al (2003) Long-term efficacy and safety of infliximab in the treatment of ankylosing spondylitis: an open, observational, extension study of a three-month, randomized, placebo-controlled trial. Arthritis Rheum 48:2224–2233
Navarra SV, Tang BF, Lu L, Lu LF, Lin H-Y et al (2014) Risk of tuberculosis with anti-tumor necrosis factor-alpha therapy: substantially higher number of patients at risk in Asia. Int J Rheum Dis 17:291–298
Song IH, Althoff CE, Haibel H et al (2012) Frequency and duration of drug-free remission after 1 year of treatment with etanercept versus sulfasalazine in early axial spondyloarthritis: 2 year data of the ESTHER trial. Ann Rheum Dis 71:1212–1215
Arends S, Brouwer E, van der Veer E et al (2011) Baseline predictors of response and discontinuation of tumor necrosis factor-alpha blocking therapy in ankylosing spondylitis: a prospective longitudinal observational cohort study. Arthritis Res Ther 13:R94
Rudwaleit M, Listing J, Brandt J et al (2004) Prediction of a major clinical response (BASDAI 50) to tumour necrosis factor alpha blockers in ankylosing spondylitis. Ann Rheum Dis 63:665–670
Acknowledgements
Supported by 5010 Subject (2007) of Sun Yat-sen University (Grant No. 2007023), National Natural Science Foundation for the Youth NSFY of China (Grant No. 81302583), Guangdong Natural Science Funds for Distinguished Young Scholar (Grant No. 2014A030306039), high-level personnel of special support program for Technology Innovative Talents and the Top Young of Guangdong Province (Grant No. 2015TQ01R516), the Fundamental Research Funds for the Central Universities (16ykzd05), Distinguished Young Scholar Candidates Programme for The Third Affiliated Hospital of Sun Yat-sen University, and the Pearl River Nova Program of Guangzhou (Grant No. 201610010005).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosures
None.
Rights and permissions
About this article
Cite this article
Zhao, M., Zhang, P., Fang, L. et al. Possible predictors for relapse from etanercept discontinuation in ankylosing spondylitis patients in remission: a three years’ following-up study. Clin Rheumatol 37, 87–92 (2018). https://doi.org/10.1007/s10067-017-3763-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-017-3763-x