Abstract
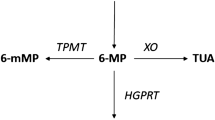
6-Thioguanine nucleotide (6-TGN) is the active metabolite of thiopurine drugs azathioprine and 6-mercaptopurine. 6-Methylmercaptopurine (6-MMP) is an inactive and potentially hepatotoxic metabolite. A subgroup of patients (shunters) preferentially produce 6-MMP instead of 6-TGN, therefore displaying thiopurine resistance and risk for hepatotoxicity. Outside inflammatory bowel disease literature, few data exist regarding individualized thiopurine therapy based on metabolite monitoring. This study sought to describe metabolite monitoring in patients receiving weight-based thiopurine for systemic autoimmune diseases. Patients were enrolled using a laboratory database, and data were retrospectively collected. The correlation between the highest thiopurine dose (mg/kg) and the 6-TGN concentration (pmol/8 × 108 erythrocytes) was estimated with Pearson’s correlation coefficient. Seventy-one patients with various systemic autoimmune conditions were enrolled. The correlation between the thiopurine dose and the 6-TGN level was weak for the overall patient sample (r = 0.201, p = 0.092) and for the subgroup of non-shunters (r = 0.278, p = 0.053). Subjects with 6-MMP levels >5700 pmol/8 × 108 erythrocytes had more hepatic cytolysis compared to subjects with 6-MMP <5700, OR = 4.36 (CI 95% 1.18–16.13, p = 0.027). Twenty-two patients (31%) were identified as shunters. Six shunters developed hepatotoxicity, five of which had 6-MMP concentration >5700. Eleven non-shunters had hepatotoxicity, one of which had 6-MMP >5700. Thiopurine metabolite monitoring shows wide variability in 6-TGN levels among patients treated with weight-based thiopurine for systemic autoimmune diseases. Thirty-one percent of the patients in our series fulfilled the shunter definition. Thiopurine metabolite monitoring and dose adjustment to improve maintenance of remission and avoid hepatotoxicity should be studied prospectively.



Similar content being viewed by others
References
Houssiau FA, Cruz DD, Sangle S, Remy P, Vasconcelos C, Petrovic R et al (2010) Azathioprine versus mycophenolate mofetil for long-term immunosuppression in lupus nephritis: results from the MAINTAIN nephritis trial. Ann Rheum Dis 69:2083–2089. doi:10.1136/ard.2010.131995
Croyle L, Hoi A, Morand EF (2015) Characteristics of azathioprine use and cessation in a longitudinal lupus cohort. Lupus Sci Med 2:e000105–e000110. doi:10.1136/lupus-2015-000105
Pagnoux C, Mahr A, Hamidou MA, Boffa JJ, Ruivard M, Ducroix JP et al (2008) Azathioprine or methotrexate maintenance for ANCA-associated vasculitis. N Engl J Med 359:2790–2803. doi:10.1056/NEJMoa0802311
Guillevin L, Pagnoux C, Karras A, Khouatra C, Aumaître O, Cohen P et al (2014) Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med 371:1771–1780. doi:10.1056/NEJMoa1404231
Chouchana L, Narjoz C, Beaune P, Loriot MA, Roblin X (2012) Review article: the benefits of pharmacogenetics for improving thiopurine therapy in inflammatory bowel disease. Aliment Pharmacol Ther 35:15–36. doi:10.1111/j.1365-2036.2011.04905.x
Amin J, Huang B, Yoon J, Shih DQ (2015) Update 2014: advances to optimize 6-mercaptopurine and azathioprine to reduce toxicity and improve efficacy in the management of IBD. Inflamm Bowel Dis 21:445–452. doi:10.1097/MIB.0000000000000197
Doré M, Frenette AJ, Mansour AM, Troyanov Y, Bégin J (2014) Febuxostat as a novel option to optimize thiopurines’ metabolism in patients with inadequate metabolite levels. Ann Pharmacother 48:648–651. doi:10.1177/1060028014521389
Blaker PA, Arenas-Hernandez M, Smith M, Shobowale-Bakre EM, Fairbanks L, Irving PM et al (2013) Mechanism of allopurinol induced TPMT inhibition. Biochem Pharmacol 86:539–547. doi:10.1016/j.bcp.2013.06.002
Haines ML, Ajlouni Y, Irving PM, Sparrow MP, Rose R, Gearry RB et al (2011) Clinical usefulness of therapeutic drug monitoring of thiopurines in patients with inadequately controlled inflammatory bowel disease. Inflamm Bowel Dis 17:1301–1307. doi:10.1002/ibd.21458
Sahasranaman S, Howard D, Roy S (2008) Clinical pharmacology and pharmacogenetics of thiopurines. Eur J Clin Pharmacol 64:753–767. doi:10.1007/s00228-008-0478-6
Cuffari C, Théorêt Y, Latour S, Seidman EG (1996) 6-mercaptopurine metabolism in Crohn’s disease: correlation with efficacy and toxicity. Gut 39:401–406. doi:10.1136/gut.39.3.401
Weinshilboum RM, Sladek SL (1980) Mercaptopurine pharmacogenetics: monogenic inheritance of erythrocyte thiopurine methyltransferase activity. Am J Hum Genet 32:651–662
Gisbert JP, Gomollón F (2008) Thiopurine-induced myelotoxicity in patients with inflammatory bowel disease: a review. Am J Gastroenterol 103:1783–1800. doi:10.1111/j.1572-0241.2008.01848.x
Gisbert JP, Niño P, Rodrigo L, Cara C, Guijarro LG (2006) Thiopurine methyltransferase (TPMT) activity and adverse effects of azathioprine in inflammatory bowel disease: long-term follow-up study of 394 patients. Am J Gastroenterol 101:2769–2776. doi:10.1111/j.1572-0241.2006.00843.x
Seidman EG (2003) Clinical use and practical application of TPMT enzyme and 6-mercaptopurine metabolite monitoring in IBD. Rev Gastroenterol Disord 3:S30–S38
Lichtenstein GR (2004) Use of laboratory testing to guide 6-mercaptopurine/azathioprine therapy. Gastroenterology 127:1558–1564. doi:10.1053/j.gastro.2004.09.061
Smith M, Blaker P, Patel C, Marinaki A, Arenas M, Escuredo E et al (2013) The impact of introducting thioguanine nucleotide monitoring into an inflammatory bowel disease clinic. Int J Clin Pract 67:161–169. doi:10.1111/ijcp.12039
Dubinski MC, Lamothe S, Yang HY, Targan SR, Sinnett D, Théorêt Y et al (2000) Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology 118:705–713. doi:10.1053/gg.2000.5925
Osterman MT, Kundu R, Lichtenstein GR, Lewis JD (2006) Association of 6-thioguanine nucleotide levels and inflammatory bowel disease activity: a meta-analysis. Gastroenterology 130:1047–1053. doi:10.1053/j.gastro.2006.01.046
Gilissen LPL, Wong DR, Engels LGJB, Bierau J, Bakker JA, Paulussen ADC et al (2012) Therapeutic drug monitoring of thiopurine metabolites in adult thiopurine tolerant IBD patients on maintenance therapy. J Crohns Colitis 6:698–707. doi:10.1016/j.crohns.2011.12.003
Moreau AC, Paul S, Tedesco ED, Rinaudo-Gaujous M, Boukhadra N, Genin C et al (2014) Association between 6-thioguanine nucleotides levels and clinical remission in inflammatory disease: a meta-analysis. Inflamm Bowel Dis 20:464–471. doi:10.1097/01.MIB.0000439068.71126.00
Dhaliwal HK, Anderson R, Thornhill EL, Schneider S, Mcfarlane E, Gleeson D et al (2012) Clinical significance of azathioprine metabolites for the maintenance of remission in autoimmune hepatitis. Hepatology 56:1401–1408. doi:10.1002/hep.25760
Gearry RB, Barclay ML (2005) Azathioprine and 6-mercaptopurine pharmacogenetics and metabolite monitoring in inflammatory bowel disease. J Gastroenterol Hepatol 20:1149–1157. doi:10.1111/j.1400-1746.2005.03832.x
Dubinski MC, Yang HY, Hassard PV, Seidman EG, Kam LY, Abreu MT et al (2002) 6-MP metabolite profiles provide a biochemical explanation for 6-MP resistance in patients with inflammatory bowel disease. Gastroenterology 122:904–915. doi:10.1053/gast.2002.32420
Cuffari C, Dassopoulos T, Turnbough L, Thompson RE, Bayless TM (2004) Thiopurine methyltransferase activity influences clinical response to azathioprine in inflammatory bowel disease. Clin Gastroenterol Hepatol 2:410–417
Ansari A, Hassan C, Dudley J, Marinaki A, Shobowale-Bakre EM, Seed P et al (2002) Thiopurine methyltransferase activity and the use of azathioprine in inflammatory bowel disease. Aliment Pharmacol Ther 16:1743–1750. doi:10.1046/j.0269-2813.2002.01353.x
van Egmond R, Chin P, Zhang M, Sies CW, Barclay ML (2012) High TPMT enzyme activity does not explain drug resistance due to preferential 6-mercaptopurine production in patients on thiopurine treatment. Aliment Pharmacol Ther 35:1181–1189. doi:10.1111/j.1365-2036.2012.05084.x
Sparrow MP, Hande SA, Friedman S, Cao D, Hanauer SB (2007) Effect of allopurinol on clinical outcomes in inflammatory bowel disease nonresponders to azathioprine or 6-mercaptopurine. Clin Gastroenterol Hepatol 5:209–214. doi:10.1016/j.cgh.2006.11.020
Gardiner SJ, Gearry RB, Burt MJ, Chalmers-Watson T, Chapman BA, Ross AG et al (2011) Allopurinol might improve response to azathioprine and 6-mercaptopurine by correcting an unfavorable metabolite ratio. J Gastroenterol Hepatol 26:49–54. doi:10.1111/j.1440-1746.2010.06489.x
Seinen ML, van Asseldonk DP, de Boer NKH, Losekoot N, Smid K, Mulder CJJ et al (2013) The effect of allopurinol and low-dose thiopurine combination therapy on the activity of three pivotal thiopurine metabolizing enzymes: results form a prospective pharmacological study. J Crohns Colitis 7:812–819. doi:10.1016/j.crohns.2012.12.006
Askanase AD, Wallace DJ, Weisman MH, Tseng CE, Bernstein L, Belmont HM et al (2009) Use of pharmacogenetics, enzymatic phenotyping, and metabolite monitoring to guide treatment with azathioprine in patients with systemic lupus erythematosus. J Rheumatol 36:89–95. doi:10.3899/jrheum.070968
Ford LT, Berg JD (2003) Determination of thiopurine S-methyltransferase activity in erythrocytes using 6-thioguanine as substrate and a non-extraction liquid chromatographic technic. J Chromatogr 798:111–115. doi:10.1016/j.jchromb.2003.09.017
Ford LT, Cooper SC, Lewis MJ, Berg JD (2004) Reference intervals for thiopurine S-methyltransferase activity in red blood cells using 6-thioguanine as substrate and rapid non-extraction liquid chromatography. Ann Clin Biochem 41:303–308. doi:10.1258/0004563041201617
Lennard L, Singleton HJ (1992) High-performance liquid chromatographic assay of the methyl and nucleotide metabolites of 6-mercaptopurine: quantitation of red blood cell 6-thioguanine nucleotide, 6-thioinosinic acid and 6-methylmercaptopurine metabolites in a single sample. J Chromatogr 583:83–90
Smith MA, Blaker P, Marikani AM, Anderson SH, Irving PM, Sanderson JD (2012) Optimising outcome on thiopurines in inflammatory bowel disease by co-prescription of allopurinol. J Crohns Colitis 6:905–912. doi:10.1016/j.crohns.2012.02.007
Dubinski MC, Reyes E, Ofman J, Chiou CF, Wade S, Sandborn WJ (2005) A cost-effectiveness analysis of alternative disease management strategies in patients with Crohn’s disease treated with azathioprine or 6-mercaptopurine. Am J Gastroenterol 100:2239–2247. doi:10.1111/j.1572-0241.2005.41900.x
Acknowledgement
We are grateful to Maxime Rhéaume, MD, for the critical review of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Institutional research ethics committee approved the study protocol, which did not imply clinical experiments on human subjects. In the context of a retrospective design, patient’s formal consent was not applicable.
Disclosures
None.
Funding
None.
Rights and permissions
About this article
Cite this article
Chapdelaine, A., Mansour, AM., Troyanov, Y. et al. Metabolite monitoring to guide thiopurine therapy in systemic autoimmune diseases. Clin Rheumatol 36, 1341–1348 (2017). https://doi.org/10.1007/s10067-017-3554-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-017-3554-4