Abstract
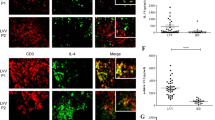
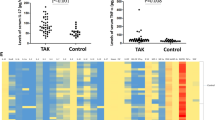
Takayasu’s arteritis (TAK) is a type of large vessel vasculitis which involves the aorta and its major branches. Interleukin (IL)-9 or IL-9-producing Th9 cells were found to be involved in pathogenesis of autoimmune arteritis such as giant cell arteritis, but IL-9 or Th9 cells in TAK were not well known. Here, this study aims to analyze the levels of serum IL-9 and their major source Th9 cells in TAK. With the help of cytometric bead array (CBA), a total of 21 patients with TAK were examined for serum levels of cytokines IL-4, IL-6, IL-8, IL-17, IL-10, TNF-α, IFN-γ, and IL-9. Flow cytometry techniques were used to examine the frequencies of Th9 cells from peripheral blood mononuclear cells (PBMCs) for 11 patients with active TAK and 10 healthy controls. Higher serum levels of serum IL-6 (P < 0.05), TNF-α (P < 0.05), and IL-9 level (P < 0.05) were observed in TAK patients compared to those of healthy controls. Higher frequencies of CD4+ IL-9+ T cells and CD4+ PU.1+ T cells in PBMCs and IL-9+ PU.1+ T cells in CD4+ T cells were observed in active TAK patients than those in healthy controls (all P < 0.01). The levels of IL-9 had a positive correlation with ESR (r = 0.975, P = 0.015) in these cases. Our data suggested that Th9 cells and IL-9 could possibly be involved in the pathogenesis of TAK.


Similar content being viewed by others
References
Vaideeswar P, Deshpande JR (2013) Pathology of Takayasu arteritis: a brief review. Ann Pediatr Cardiol 6(1):52–58
de Souza AW, de Carvalho JF (2014) Diagnostic and classification criteria of Takayasu arteritis. J Autoimmun 48-49:79–83
Seko Y, Minota S, Kawasaki A, Shinkai Y, Maeda K, Yagita H, Okumura K, Sato O, Takagi A, Tada Y, et al. (1994) Perforin-secreting killer cell infiltration and expression of a 65-kD heat-shock protein in aortic tissue of patients with Takayasu’s arteritis. J Clin Invest 93(2):750–758
Sagar S, Ganguly NK, Koicha M, Sharma BK (1992) Immunopathogenesis of Takayasu arteritis. Heart Vessels Suppl 7:85–90
Arnaud L, Haroche J, Mathian A, Gorochov G, Amoura Z (2011) Pathogenesis of Takayasu’s arteritis: a 2011 update. Autoimmun Rev 11(1):61–67
Alibaz-Oner F, Yentur SP, Saruhan-Direskeneli G, Direskeneli H (2015) Serum cytokine profiles in Takayasu’s arteritis: search for biomarkers. Clin Exp Rheumatol 33(2 Suppl 89):32–35
Park MC, Lee SW, Park YB, Lee SK (2006) Serum cytokine profiles and their correlations with disease activity in Takayasu’s arteritis. Rheumatology (Oxford) 45(5):545–548
Mariani N, A S, Aubry-Rozier B (2013) Two cases of Takayasu’s arteritis occurring under anti-TNF therapy. Joint Bone Spine 80(2):211–213
Tripathy NK, Chauhan SK, Nityanand S (2004) Cytokine mRNA repertoire of peripheral blood mononuclear cells in Takayasu’s arteritis. Clin Exp Immunol 138(2):369–374
Sun Y, Ma L, Yan F, Liu H, Ding Y, Hou J, Jiang L (2012) MMP-9 and IL-6 are potential biomarkers for disease activity in Takayasu’s arteritis. Int J Cardiol 156(2):236–238
Kaplan MH (2013) Th9 cells: differentiation and disease. Immunol Rev 252(1):104–115
Ramming A, Druzd D, Leipe J, Schulze-Koops H, Skapenko A (2012) Maturation-related histone modifications in the PU.1 promoter regulate Th9-cell development. Blood 119(20):4665–4674
Hoppenot D, Malakauskas K, Lavinskiene S, Bajoriuniene I, Kalinauskaite V, Sakalauskas R (2015) Peripheral blood Th9 cells and eosinophil apoptosis in asthma patients. Medicina (Kaunas) 51(1):10–17
Ciccia F, Guggino G, Rizzo A, Manzo A, Vitolo B, La Manna MP, Giardina G, Sireci G, Dieli F, Montecucco CM et al (2015) Potential involvement of IL-9 and Th9 cells in the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford)
Kara EE, Comerford I, Bastow CR, Fenix KA, Litchfield W, Handel TM, McColl SR (2013) Distinct chemokine receptor axes regulate Th9 cell trafficking to allergic and autoimmune inflammatory sites. J Immunol 191(3):1110–1117
Xu H, Rizzo LV, Silver PB, Caspi RR (1997) Uveitogenicity is associated with a Th1-like lymphokine profile: cytokine-dependent modulation of early and committed effector T cells in experimental autoimmune uveitis. Cell Immunol 178(1):69–78
Essa MM, Subash S, Akbar M, Al-Adawi S, Guillemin GJ (2015) Long-term dietary supplementation of pomegranates, figs and dates alleviate neuroinflammation in a transgenic mouse model of Alzheimer’s disease. PLoS One 10(3):e0120964
Saresella M, Calabrese E, Marventano I, Piancone F, Gatti A, Alberoni M, Nemni R, Clerici M (2011) Increased activity of Th-17 and Th-9 lymphocytes and a skewing of the post-thymic differentiation pathway are seen in Alzheimer’s disease. Brain Behav Immun 25(3):539–547
Ouyang H, Shi Y, Liu Z, Feng S, Li L, Su N, Lu Y, Kong S (2013) Increased interleukin9 and CD4+ IL-9+ T cells in patients with systemic lupus erythematosus. Mol Med Rep 7(3):1031–1037
Schlapbach C, Gehad A, Yang C, Watanabe R, Guenova E, Teague JE, Campbell L, Yawalkar N, Kupper TS, Clark RA (2014) Human TH9 cells are skin-tropic and have autocrine and paracrine proinflammatory capacity. Sci Transl Med 6(219):219ra218
Ciccia F, Rizzo A, Guggino G, Cavazza A, Alessandro R, Maugeri R, Cannizzaro A, Boiardi L, Iacopino DG, Salvarani C, et al. (2015) Difference in the expression of IL-9 and IL-17 correlates with different histological pattern of vascular wall injury in giant cell arteritis. Rheumatology (Oxford) 54(9):1596–1604
Arend WP, Michel BA, Bloch DA, Hunder GG, Calabrese LH, Edworthy SM, Fauci AS, Leavitt RY, Lie JT, Lightfoot RW Jr, et al. (1990) The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum 33(8):1129–1134
Kerr GS, Hallahan CW, Giordano J, Leavitt RY, Fauci AS, Rottem M, Hoffman GS (1994) Takayasu arteritis. Ann Intern Med 120(11):919–929
Arnaud L, Kahn JE, Girszyn N, Piette AM, Bletry O (2006) Takayasu’s arteritis: an update on physiopathology. Eur J Intern Med 17(4):241–246
Ye ZJ, Yuan ML, Zhou Q, RH D, Yang WB, Xiong XZ, Zhang JC, Wu C, Qin SM, Shi HZ (2012) Differentiation and recruitment of Th9 cells stimulated by pleural mesothelial cells in human Mycobacterium tuberculosis infection. PLoS One 7(2):e31710
Goswami R, Kaplan MH (2012) Gcn5 is required for PU.1-dependent IL-9 induction in Th9 cells. J Immunol 189(6):3026–3033
Kaplan MH, Hufford MM, Olson MR (2015) The development and in vivo function of T helper 9 cells. Nat Rev Immunol 15(5):295–307
Froidure A, Shen C, Gras D, Van Snick J, Chanez P, Pilette C (2014) Myeloid dendritic cells are primed in allergic asthma for thymic stromal lymphopoietin-mediated induction of Th2 and Th9 responses. Allergy 69(8):1068–1076
Gerlach K, Hwang Y, Nikolaev A, Atreya R, Dornhoff H, Steiner S, Lehr HA, Wirtz S, Vieth M, Waisman A, et al. (2014) TH9 cells that express the transcription factor PU.1 drive T cell-mediated colitis via IL-9 receptor signaling in intestinal epithelial cells. Nat Immunol 15(7):676–686
Nalleweg N, Chiriac MT, Podstawa E, Lehmann C, Rau TT, Atreya R, Krauss E, Hundorfean G, Fichtner-Feigl S, Hartmann A, et al. (2015) IL-9 and its receptor are predominantly involved in the pathogenesis of UC. Gut 64(5):743–755
Ma L, Xue HB, Guan XH, Shu CM, Zhang JH, Yu J (2014) Possible pathogenic role of T helper type 9 cells and interleukin (IL)-9 in atopic dermatitis. Clin Exp Immunol 175(1):25–31
Yang J, Li Q, Yang X, Li M (2015) Interleukin-9 is associated with elevated anti-double-stranded DNA antibodies in lupus-prone mice. Mol Med 21:364–370
Ding X, Cao F, Cui L, Ciric B, Zhang GX, Rostami A (2015) IL-9 signaling affects central nervous system resident cells during inflammatory stimuli. Exp Mol Pathol
Acknowledgments
We thank Professor Jian-ping Guo for her help in revising the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
ᅟ
Ten healthy subjects who were matched in gender and age without any records of infection and chronic diseases such as tumor, atherosclerosis, and allergic disease were selected from the Health Care Center of An Zhen Hospital with the approval of the Ethical Committee.
Funding
This project was supported by grants from the National Natural Science Foundation of China (81400361) and Basic-clinical Cooperation Foundation of Capital Medical University (14JL164). The sponsors had no role in the study design, data collection and analysis, decision to publish, or preparation.
Disclosures
None.
Rights and permissions
About this article
Cite this article
Pan, Ll., Du, J., Gao, N. et al. IL-9-producing Th9 cells may participate in pathogenesis of Takayasu’s arteritis. Clin Rheumatol 35, 3031–3036 (2016). https://doi.org/10.1007/s10067-016-3399-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-016-3399-2