Abstract
The aim of this study was to investigate treatment response and hepatic safety of anti-tumor necrosis factor-α therapy among patients with concomitant rheumatoid arthritis (RA) and hepatitis C virus (HCV) infection. We reviewed the charts of 101 consecutive RA patients who were eligible for anti-TNF-α therapy in the Chiayi Branch of Chang Gung Memorial Hospital. Group A patients were sero-positive for anti-HCV antibodies and had HCV RNA but were negative for hepatitis B surface antigen (HBsAg). Group B (the control group) patients were sero-negative for both anti-HCV antibodies and HBsAg. Response to anti-TNF-α treatment was assessed by calculating disease activity score at 28 joints (DAS28) at baseline and 5, 8, and 11 months after the start of TNF-α antagonist therapy. Percentage change in DAS28 from baseline to month 5 was 21.36 ± 8.01 % in group A and 26.98 ± 10.43 % in group B (p = 0.011). However, there was no obvious difference in treatment response between groups at other time points. Anti-TNF-α therapy was discontinued within 1 year of starting treatment in two subjects in group A and 4 in group B. Response to anti-TNF-α was better in group B than in group A at 5 months, but there was no substantial difference in response at the 1-year evaluation. Although the study sample was small, our results suggest that the safety of anti-TNF-α therapy is similar in RA patients with and without concomitant HCV infection.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disorder of unknown etiology that primarily involves the joints and requires long-term treatment [1]. Affected joints exhibit hyperplasia of inflamed synovium infiltrated with immune cells, which release cytokines such as tumor necrosis factor-α (TNF-α) [2]. In the past, the first-line treatment for RA was disease-modifying anti-rheumatic drugs (DMARDs), which were given as early as possible in the disease process to suppress disease activity [3]. Since the late 1990s, the success of biologic therapy has changed RA treatment. TNF-α antagonists are biologic immunomodulators used to treat a number of inflammatory conditions, including RA, spondyloarthritis, psoriatic arthritis, and inflammatory bowel disease [4]. The development of anti-TNF-α therapy has improved outcomes for many patients. However, several adverse effects of anti-TNF-α therapy have been identified, including infection, malignancies, lupus-like syndrome, and demyelinating neuropathy.
The American College of Rheumatology recommends that TNF-α inhibitors should not be given to RA patients with acute or chronic hepatitis B or C infection (treated or untreated) with significant liver injury, defined as chronic Child-Pugh class B or C disease [5]. The effects of TNF-α inhibition on viral infection might vary with the specific type of viral infection. Inhibition of TNF-α appears to delay clearance of hepatitis B, and this effect is controlled by cytokine and cellular mechanisms [6]. However, with the availability of TNF inhibitors, the safety and efficacy of blocking TNF in patients with chronic viral infections must be carefully evaluated. Although some RA patients with concomitant RA and hepatitis C virus (HCV) infection are undertreated due to concerns regarding possible worsening of HCV viremia and deterioration of liver function, most studies show that TNF-α antagonists are safe when given to such patients. Indeed, serum and hepatic TNF-α levels are increased in patients with HCV, and inhibition of TNF might thus be beneficial in HCV [7].
In addition to challenges related to HCV infection, the efficacy of TNF-α antagonists for treating RA in individuals with HCV is a concern. Although phase II and III clinical trials showed that TNF-α antagonists resulted in adequate response among patients with RA, these trials always excluded individuals with HCV infection [8–12]. There are only limited data on the safety and efficacy of TNF-α antagonist therapy for patients with concomitant RA and HCV infection. Anti-TNF-α treatment for concurrent rheumatoid arthritis and hepatitis C virus infection was investigated in small population studies [13]. However, previous studies lacked control groups. In the present study, we compared treatment response and recorded causes of early withdrawal from TNF-α antagonist therapy during a 1-year treatment period in RA patients with and without concomitant HCV infection.
Materials and methods
Study design
In this retrospective case series study, we investigated the clinical effects of TNF-α antagonist therapy for RA patients with and without concomitant HCV infection by reviewing the medical records of 101 patients with RA who were regularly followed up in the Rheumatology Department of the Chiayi Branch of Chang Gung Memorial Hospital from January 2003 through October 2011. Eligible patients were older than 18 years and had RA diagnosed according to the revised 1987 American College of Rheumatology (ACR) criteria [14]. Only patients with active RA who were suitable candidates for anti-TNF-α therapy were included. Active RA was defined as a disease activity score at 28 joints (DAS28) greater than 5.1 after at least 6 months of treatment with at least 2 DMARDs at maximum dose. In addition, at least 1 of the DMARDs had to be methotrexate (MTX) at a maximum dose of 15 mg/week, otherwise, side effects of MTX developed. The study protocol was approved by the Chang Gung Memorial Hospital Research Ethics Committee.
Patient selection
HCV and hepatitis B infection were detected by anti-HCV antibody and hepatitis B surface antigen (HBsAg) testing, respectively. HCV infection was confirmed when HCV RNA was detected. Patients with coexisting RA and hepatitis B infection were excluded, as were those who had a positive anti-HCV antibody test result but no HCV RNA. Patients with acute hepatitis were also excluded. No patient with concomitant RA and HCV infection received antiviral therapy during anti-TNF-α therapy. Detection of HBsAg, anti-HCV antibody, and HCV RNA was done by enzyme immunoassay (Roche Molecular), COBAS AmpliPrep/COBAS TaqMan assay (Roche Molecular), and real-time polymerase chain reaction (PCR; Abbott Diagnostics), respectively. The upper limit of normal (ULN) of liver function was 36 IU/ml for alanine aminotransferase (ALT) and 34 IU/ml for aspartate transaminase (AST). Liver injury was defined as an elevation in AST or ALT level greater than three times the ULN [15, 16]. The reference range for rheumatoid factor (RF) was 0 to 20 IU/ml. Patients who were positive for anti-HCV antibodies and had HCV RNA were classified as the RA-HCV group, while those who were sero-negative for both anti-HCV antibodies and HBsAg were classified as the control group.
Outcome measures
To achieve and maintain disease remission, patients received etanercept (25 mg twice a week) or adalimumab (40 mg every 2 weeks). Patients who switched TNF-α antagonists were also included. Clinical assessment of patients included DAS28, erythrocyte sedimentation rate (ESR), hemogram, renal function, and serum transaminase levels. The baseline characteristics assessed included age, sex, disease duration, age at RA onset, and age at first use of TNF-α antagonist. Outcome measures were evaluated at months 5, 8, and 11. Concomitant immunosuppressive treatment was MTX, sulfasalazine (SSZ), hydroxychloroquine (HCQ), leflunomide (LEF), cyclosporin A (CsA), and azathioprine (AZA). Use of non-steroidal anti-inflammatory drugs (NSAIDs) and steroids was also analyzed.
Statistical analysis
Patients who received at least one injection of etanercept or adalimumab during any period (the intention-to-treat [ITT] population) were included in the response analyses. The analyses were performed on the ITT population, which included data from all patients who discontinued treatment for any reason. Differences between the two groups were analyzed with the Mann–Whitney U test or χ 2 test, as appropriate. The mean and standard deviation (SD) are presented for continuous variables. Frequency and percentage are given for categorical variables. A repeated-measures design was used; DAS28 was evaluated at baseline, 5, 8, and 11 months throughout the duration of TNF-α antagonist therapy. SPSS version 18.0 was used for all statistical analyses.
Results
Patient enrollment and disposition
In this retrospective study, 138 patients with active RA treated with TNF inhibitors were regularly followed up in the Rheumatology Department of the Chiayi Branch of Chang Gung Memorial Hospital from January 2003 through October 2011 (Fig. 1). Among these patients, 17 were excluded due to missing data on hepatitis B or C status, and nine were excluded because of HBsAg positivity. Thirty patients were positive for anti-HCV antibody, and 20 of the 27 patients who underwent HCV RNA testing were positive. In addition, 81 patients with RA were negative for HBsAg and anti-HCV antibodies. Ultimately, we analyzed data from 20 patients with concomitant RA and HCV (group A) and 81 with RA only (group B).
Baseline characteristics
The baseline characteristics of the 101 patients with RA (20 in group A and 81 in group B) are shown in Table 1. Mean age at RA onset significantly differed: 58.3 ± 9.4 years in group A vs 52.1 ± 13 years in group B (p = 0.029). Mean age at the start of anti-TNF-α therapy also significantly differed (p = 0.025). However, the interval between RA onset and use of TNF-α antagonist was similar between groups. The frequency of RF positivity and RF titer was higher in group A, although not significantly so. Before the use of a TNF-α antagonist, DAS28 was lower in group B than in group A: 5.90 ± 0.51 and 6.31 ± 0.74, respectively (p = 0.018). ALT level was higher in group A than in group B, but the frequency of liver injury before anti-TNF-α therapy was similar between groups. MTX was the most frequently used (85.2 %) DMARD in group B and, as expected, was more frequently used than in group A. In addition, CsA was more frequently given to patients in group A than to those in group B (p = 0.043). Regarding anti-TNF therapy, 18 (85.7 %) patients in group A were given etanercept, and two were given adalimumab. In group B, 59 (73.8 %) patients were given etanercept, and 22 were given adalimumab (data not shown in Table 1).
Changes in mean clinical indicators
At baseline, ALT level was 31.2 ± 22.8 in group A and 22.5 ± 22.3 IU/ml in group B (p = 0.035). During 1 year of anti-TNF-α therapy, 3 of the 101 patients developed liver injury: 1 (1.23 %) patient in group B and 2 (10 %) patients in group A (p = 0.099 by Fisher’s exact test). Liver function later normalized in all three patients. After MTX was temporarily stopped, serum liver transaminase improved in the single patient in group B. One patient in group A was closely monitored, and anti-TNF-α therapy was paused for 1 month for the other patient.
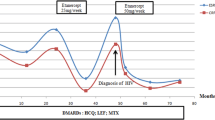
DAS28 values were 6.31 ± 0.74 at baseline, 4.97 ± 0.78 at 5, 3.92 ± 0.78 at 8, and 3.54 ± 1.0 at 11 months in group A; 5.90 ± 0.51 at baseline, 4.31 ± 0.75 at 5, 3.54 ± 0.77 at 8, and 3.49 ± 1.07 at 11 months in group B (Fig. 2). DAS28 was lower in group B than in group A at baseline (p = 0.018), month 5 (p = 0.002), and month 8 (p = 0.029), but was similar at month 11. Changes in DAS28 from baseline were 1.35 ± 0.49 at 5, 2.4 ± 0.75 at 8, and 2.77 ± 1.16 at 11 months in group A; 1.58 ± 0.62 at 5, 2.36 ± 0.8 at 8, and 2.43 ± 1.2 at 11 months in group B. Changes in DAS28 significantly differed between groups only at month 5 (p = 0.037). In addition, percentage changes in DAS28 from baseline were 21.36 ± 8.01 % at 5, 37.81 ± 11.49 % at 8, and 43.42 ± 18.12 % at 11 months in group A; 26.98 ± 10.43 % at 5, 39.92 ± 10.99 % at 8, and 40.61 ± 19.68 % at 11 months in group B. The difference between groups was significant at month 5 (p = 0.011) but not at month 8 (p = 0.114) or 11 (p = 0.597). ESR did not significantly differ between groups at baseline (43.2 ± 21.8 mm/h in group A vs 49.9 ± 19.6 mm/h in group B), month 5 (28.2 ± 18.7 mm/h vs 37.5 ± 27.0 mm/h), month 8 (27.7 ± 20.9 mm/h vs 35.6 ± 27.3 mm/h), or month 11 (27.3 ± 22.9 mm/h vs 33.8 ± 23.1 mm/h) of TNF-α antagonist therapy. Using a repeated-measures design and analysis of covariates in Table 1, including age at RA onset and age at start of TNF-α antagonist therapy and presence of HCV infection, presence of HCV infection was significantly associated with the time course of DAS28 in the two groups.
Changes in mean clinical indices in groups A and B. a DAS28, b percentage change in DAS28 from baseline, c ESR, d number of patients at each visit. Analysis of covariance (ANCOVA) models was used for statistical comparisons between groups at all time points. DAS28 values are based on the intention-to-treat population of patients receiving anti-TNF-α therapy with data available at a visit of interest. DAS28 disease activity score based (28 joints), ESR erythrocyte sedimentation rate. *p < 0.05; **p < 0.01
Six patients withdrew from anti-TNF-α therapy, and number of withdrawals did not significantly differ between groups. Three of the six patients discontinued anti-TNF-α therapy due to intolerance (two had an allergic reaction and one developed colon cancer), one was a non-responder, one was lost to follow-up, and one patient with disease remission was denied coverage for anti-TNF-α therapy by the National Health Insurance (NHI) system in Taiwan. Eighteen (90 %) of 20 patients in group A and 77 (95.1 %) of 81 patients in group B were observed for 1 year of TNF-α antagonist therapy (Fig. 2). The drug survival rate was similar between groups.
Discussion
In this study, we investigated 101 patients with documented RA who were submitted to a 12-month anti-TNF-α therapy course and the results showed that there was no obvious difference in treatment response in RA patients with and without concomitant HCV infection. Moreover, the number of withdrawals did not significantly differ between RA patients with and without concomitant HCV infection. The results match the medical literature data. Previous studies showed that anti-TNF-α therapy had adequate safety of RA patients concomitant HCV infection, particularly with regard to viremia and hepatotoxicity, and did not result in serious adverse events [17–24]. However, only a few of these studies reported overall response rates and none reported response pattern [18, 19]. In addition, none of these studies included a control group to evaluate treatment effectiveness (Table 2). This is the first controlled study to compare effectiveness and hepatic safety among patients with RA only and concomitant RA and hepatitis C. After 1 year of treatment, clinical response to anti-TNF-α therapy was similar between these two patient groups. During anti-TNF-α therapy, DAS28 and percentage change in DAS28 from baseline were better at month 5 in group B as compared with group A. However, after 1 year of follow-up, DAS28 and percentage change in DAS28 were both similar between groups. This is a new finding regarding response to anti-TNF-α therapy among patients with RA and concomitant HCV infection.
Recent case reports and case series studies found that TNF-α antagonists were safe when used to treat RA in individuals with coexisting HCV infection [17–25]. In our study, three patients had serum liver transaminase levels greater than three times the ULN during the 1-year treatment period. In such cases, we discontinued MTX among patients receiving it. If abnormal liver function persisted, we withheld TNF-α antagonist therapy. Liver function normalized in all three patients; however, HCV viral load was not examined. These findings suggest that it is necessary to closely monitor liver function while patients with RA and HCV receive TNF-α antagonists. Prior reports found that TNF-α antagonists were safe for patients with HCV infection and that elevation of liver enzymes associated with anti-TNF-α therapy was uncommon among patients with RA [26]. Despite these cases of hepatotoxicity, the drug adherence rate at month 11 was similar in groups A and B (95.1 vs 90 %, respectively) and slightly higher than in prior studies [27, 28].
Up to 80 % of patients with hepatitis C infection will progress to chronic infection [29]. Among our patients, 30 were positive for anti-HCV antibodies. Among these patients, 27 underwent testing for HCV RNA and 20 (74.1 %) had detectable HCV RNA. In addition, age at RA onset and age at start of TNF-α antagonist were higher in the RA-HCV group than in the control group. Ferri et al. reported that mean age at RA onset in 29 patients with concomitant HCV infection was 48.6 years (they excluded two patients with juvenile rheumatoid arthritis) [19]. In our study, mean age was 58.3 ± 9.4 years. In Taiwan, catastrophic certification for RA is provided by the NHI after a patient receives a physician-confirmed diagnosis of RA. Because HCV infection and RA may have similar clinical features, application for RA catastrophic certification may be influenced by the presence of concomitant RA and HCV [30]. The difference in mean age between the present and past studies may be due to physician delays in applying for catastrophic certification for patients in the RA-HCV group. In contrast, guidance for reimbursement of anti-TNF-α therapy is more restrictive in Taiwan than in other countries. The criterion for a patient with RA to receive anti-TNF-α therapy is a DAS28 greater than 5.1 after at least 6 months of treatment with at least two DMARDs at maximum dose. In addition, at least 1 of the DMARDs must be MTX at a maximum dose of 15 mg/week. Data on disease severity and patient age may have been influenced by this reimbursement criterion.
The prevalence of serologic markers of autoimmunity was reported to be high in individuals with chronic HCV infection [31]. Of particular interest was the fact that RF was noted in up to 76 % of men and women. In our study, RF positivity was noted in 90 % of group A patients and 75 % of group B patients. RF prevalence in group B was similar to that noted in prior clinical trials of anti-TNF-α therapy for RA patients [9, 12]. However, the higher rate of RF in group A might be due to HCV itself. HCV infection can present with rheumatic manifestations indistinguishable from RA and may be another reason for the higher rate of RF [30].
Several DMARDs are contraindicated for patients with liver transaminase levels two times the ULN and those with chronic HCV infection [5]. In clinical practice, MTX is the first choice for RA therapy, and we chose to use MTX or LEF in patients with RA with coexisting HCV infection, while closely monitoring liver function. Episodes of abnormal elevation of liver enzymes were more frequent in group A than in group B before anti-TNF-α treatment. Elevation of liver transaminases may have been related to drug hepatotoxicity in combination with the effects of chronic HCV infection [32, 33].
This study provides useful information on the clinical effectiveness of anti-TNF-α therapy for patients with rheumatoid arthritis with and without concomitant HCV infection. Nevertheless, some limitations of the study should be mentioned. First, because this is a retrospective study, some unrecognized biases might have been introduced. Second, we did not monitor HCV RNA titers in all patients with elevated liver enzymes during TNF-α antagonist therapy, so we cannot determine whether abnormal liver function was related to HCV exacerbation, drug toxicity, or other reasons. Third, for safety reasons, rheumatologist prescribed TNF inhibitor to patients in relatively stable condition among RA patients with concomitant HCV infection, especially regarding hepatic status. RA patients with more severe HCV may not be involved in this study. This is also our limitation of this retrospective study even though we enrolled all RA patients treated with TNF inhibitor. Fourth, the main differences of these two groups were mean age at RA onset, mean age at start of TNF-I, and disease activity (DAS28). These differences were our limitation because of a small number of HCV infection in RA patients. Finally, some patients may have chosen to taper the dose of DMARDs in accordance with disease activity. If so, the actual dose would not be reflected in the chart review. Because of the limitation of small sample size and not routine test HCV viral load, the safety of anti-TNF agents finding may not be generalizable to other RA patients with concomitant HCV infection. Future well-designed studies should investigate whether anti-TNF-α therapy, under the same DMARD-based regimen, is equally effective for patients with RA with and without concomitant HCV infection.
In general conclusion, the outcomes of anti-TNF-α therapy were similar in RA patients with and without concomitant HCV infection during a 1-year treatment period. Clinical response was better in group B than in group A at month 5. Elevation of liver transaminases may occur in patients receiving anti-TNF-α therapy, although we observed no conclusive evidence of HCV reactivation or drug-induced hepatotoxicity, especially among patients with RA and hepatitis C. The risk of anti-TNF-α therapy appears to be low for RA patients with concomitant HCV infection. This finding is applicable to RA patients with concomitant HCV infection who tolerate methotrexate treatment well.
References
Lee DM, Weinblatt ME (2001) Rheumatoid arthritis. Lancet 358(9285):903–911
Brennan FM, McInnes IB (2008) Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest 118(11):3537–3545
Plant MJ, Williams AL, O’Sullivan MM, Lewis PA, Coles EC, Jessop JD (2000) Relationship between time-integrated C-reactive protein levels and radiologic progression in patients with rheumatoid arthritis. Arthritis Rheum 43(7):1473–1477
Geiler J, Buch M, McDermott MF (2011) Anti-TNF treatment in rheumatoid arthritis. Curr Pharm Des 17(29):3141–3154
Saag KG, Teng GG, Patkar NM et al (2008) American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum 59(6):762–784
Pasquetto V, Wieland SF, Uprichard SL, Tripodi M, Chisari FV (2002) Cytokine-sensitive replication of hepatitis B virus in immortalized mouse hepatocyte cultures. J Virol 76(11):5646–5653
Gonzalez-Amaro R, Garcia-Monzon C, Garcia-Buey L et al (1994) Induction of tumor necrosis factor alpha production by human hepatocytes in chronic viral hepatitis. J Exp Med 179(3):841–848
Breedveld FC, Weisman MH, Kavanaugh AF et al (2006) The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum 54(1):26–37
Klareskog L, van der Heijde D, de Jager JP et al (2004) Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet 363(9410):675–681
Lipsky PE, van der Heijde DM, St Clair EW et al (2000) Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-tumor necrosis factor trial in rheumatoid arthritis with concomitant therapy study group. N Engl J Med 343(22):1594–1602
Maini R, St Clair EW, Breedveld F et al (1999) Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet 354(9194):1932–1939
Weinblatt ME, Keystone EC, Furst DE et al (2000) Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum 48(1):35–45
Giannitti C, Benucci M, Caporali R et al (2009) Efficacy and safety of anti-TNF-alpha therapy combined with cyclosporine A in patients with rheumatoid arthritis and concomitant hepatitis C virus infection. Int J Immunopathol Pharmacol 22(2):543–546
Arnett FC, Edworthy SM, Bloch DA et al (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31(3):315–324
Benichou C (1990) Criteria of drug-induced liver disorders. Report of an international consensus meeting. J Hepatol 11(2):272–276
Mumoli N, Cei M, Cosimi A (2006) Drug-related hepatotoxicity. N Engl J Med 354(20):2191–2193, author reply −3
Cansu DU, Kalifoglu T, Korkmaz C (2008) Short-term course of chronic hepatitis B and C under treatment with etanercept associated with different disease modifying antirheumatic drugs without antiviral prophylaxis. J Rheumatol 35(3):421–424
Cavazzana I, Ceribelli A, Cattaneo R, Franceschini F (2008) Treatment with etanercept in six patients with chronic hepatitis C infection and systemic autoimmune diseases. Autoimmun Rev 8(2):104–106
Ferri C, Ferraccioli G, Ferrari D et al (2008) Safety of anti-tumor necrosis factor-alpha therapy in patients with rheumatoid arthritis and chronic hepatitis C virus infection. J Rheumatol 35(10):1944–1949
Li S, Kaur PP, Chan V, Berney S (2009) Use of tumor necrosis factor-alpha (TNF-alpha) antagonists infliximab, etanercept, and adalimumab in patients with concurrent rheumatoid arthritis and hepatitis B or hepatitis C: a retrospective record review of 11 patients. Clin Rheumatol 28(7):787–791
Parke FA, Reveille JD (2004) Anti-tumor necrosis factor agents for rheumatoid arthritis in the setting of chronic hepatitis C infection. Arthritis Rheum 51(5):800–804
Peterson JR, Hsu FC, Simkin PA, Wener MH (2003) Effect of tumour necrosis factor alpha antagonists on serum transaminases and viraemia in patients with rheumatoid arthritis and chronic hepatitis C infection. Ann Rheum Dis 62(11):1078–1082
Roux CH, Brocq O, Breuil V, Albert C, Euller-Ziegler L (2006) Safety of anti-TNF-alpha therapy in rheumatoid arthritis and spondylarthropathies with concurrent B or C chronic hepatitis. Rheumatology 45(10):1294–1297
Vauloup C, Krzysiek R, Greangeot-Keros L et al (2006) Effects of tumor necrosis factor antagonist treatment on hepatitis C-related immunological abnormalities. Eur Cytokine Netw 17(4):290–293
Brunasso AM, Puntoni M, Gulia A, Massone C (2011) Safety of anti-tumour necrosis factor agents in patients with chronic hepatitis C infection: a systematic review. Rheumatology 50(9):1700–1711
Sokolove J, Strand V, Greenberg JD et al (2010) Risk of elevated liver enzymes associated with TNF inhibitor utilisation in patients with rheumatoid arthritis. Ann Rheum Dis 69(9):1612–1617
Du Pan SM, Dehler S, Ciurea A et al (2009) Comparison of drug retention rates and causes of drug discontinuation between anti-tumor necrosis factor agents in rheumatoid arthritis. Arthritis Rheum 61(5):560–568
Hetland ML, Christensen IJ, Tarp U et al (2010) Direct comparison of treatment responses, remission rates, and drug adherence in patients with rheumatoid arthritis treated with adalimumab, etanercept, or infliximab: results from 8 years of surveillance of clinical practice in the nationwide Danish DANBIO registry. Arthritis Rheum 62(1):22–32
Farci P, Alter HJ, Wong D et al (1991) A long-term study of hepatitis C virus replication in non-A, non-B hepatitis. N Engl J Med 325(2):98–104
Lovy MR, Starkebaum G, Uberoi S (1996) Hepatitis C infection presenting with rheumatic manifestations: a mimic of rheumatoid arthritis. J Rheumatol 23(6):979–983
Clifford BD, Donahue D, Smith L et al (1995) High prevalence of serological markers of autoimmunity in patients with chronic hepatitis C. Hepatology 21(3):613–619
Cohen S, Cannon GW, Schiff M et al (2001) Two-year, blinded, randomized, controlled trial of treatment of active rheumatoid arthritis with leflunomide compared with Methotrexate. Utilization of leflunomide in the treatment of rheumatoid arthritis trial investigator group. Arthritis Rheum 44(9):1984–1992
Salliot C, van der Heijde D (2009) Long-term safety of methotrexate monotherapy in patients with rheumatoid arthritis: a systematic literature research. Ann Rheum Dis 68(7):1100–1104
Potential conflicts of interest
All authors have no financial or personal relationships with other people or organizations that could inappropriately influence their work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, KM., Cheng, TT., Lin, JC. et al. Tumor necrosis factor-α antagonist therapy for concomitant rheumatoid arthritis and hepatitis C virus infection: a case series study. Clin Rheumatol 34, 1039–1046 (2015). https://doi.org/10.1007/s10067-015-2962-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-015-2962-6