Abstract
This study analyzed the effects of walking freely in virtual reality (VR) compared to walking in the real-world on dynamic balance and postural control. For this purpose, nine male and twelve female healthy participants underwent standard 3D gait analysis while walking randomly in a real laboratory and in a room-scale overground VR environment resembling the real laboratory. The VR was delivered to participants by a head-mounted-display which was operated wirelessly and calibrated to the real-world. Dynamic balance and postural control were assessed with (1) the margin of stability (MOS) in the anteroposterior (AP-MOS) and mediolateral (ML-MOS) directions at initial-contact, (2) the relationship between the mediolateral center of mass (COM) position and acceleration at mid-stance with subsequent step width, (3) and trunk kinematics during the entire gait cycle. We observed increased mediolateral (ML) trunk linear velocity variability, an increased coupling of the COM position and acceleration with subsequent step width, and a decrease in AP-MOS while walking in VR but no change in ML-MOS when walking in VR. Our findings suggest that walking in VR may result in a less reliable optical flow, indicated by increased mediolateral trunk kinematic variability, which seems to be compensated by the participants by slightly reweighing sensorimotor input and thereby consciously tightening the coupling between the COM and foot placement to avoid a loss of balance. Our results are particularly valuable for future developers who want to use VR to support gait analysis and rehabilitation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Augmented, Mixed, and virtual reality (VR) technologies extend our reality by merging the virtual with the real-world to create a fully immersive experience. The global market for these technologies was worth approximately $31 billion in 2021 and is projected to rise to nearly $300 billion by 2024 with the healthcare sector being most affected by this growth (Statista 2021). Of particular interest is the use of VR as a tool for gait training and rehabilitation (Canning et al. 2020; Janeh and Steinicke 2021). Virtual reality in rehabilitation has gained traction due to its potential to enhance motivation, provide an immersive experience, and target specific gait parameters, thereby making it a valuable adjunct to traditional therapy approaches (Benham et al. 2019; Chen et al. 2021). Indeed, there is a growing application of VR for patients with gait and balance disorders such as Parkinson’s Disease (Triegaardt et al. 2020; Lu et al. 2021) Cerebral Palsy (de Oliveira et al. 2016), stroke (Palacios-Navarro and Hogan 2021; Ahn and Hwang 2019; Peng et al. 2021), amputation (Darter and Wilken 2011), and healthy aging (Lee 2020; Delgado and Der Ananian 2021; de Vries et al. 2020; Willaert et al. 2020).
In gait rehabilitation, to date, VR is primarily used on treadmills with mainly semi-immersive VR setups such as screens in front of the patients (Brepohl and Leite 2023). However, the rapidly advancing technology of head-mounted displays (HMD) offer exciting but partly unexplored possibilities in using VR to aid gait rehabilitation during overground scenarios. Head-mounted displays allow patients to naturally walk and navigate through virtual environments without being restricted to treadmills or to seeing the VR on a stationary screen. It allows them to explore and interact with their virtual environment in a highly realistic way where the physical movements (e.g., of the head and hands) are tracked and used in real-time for immersion. However, before such highly immersive overground VR environments can be used as an effective and safe tool for populations with gait and balance disorders, it is imperative to examine how VR affects dynamic balance while walking in healthy adults. This is necessary before VR can be used in other populations for testing and training dynamic balance and postural control. Specifically, to understand what the confounding effects of VR are and how they potentially alter the way our neuromuscular system maintains balance and postural control while navigating overground through VR environments.
While walking, balance is threatened as the center of mass (COM) is volitionally displaced outside the base of support (BoS) during the swing phase to progress forward (Winter 1995; Siragy and Nantel 2018). Further, even during the double-support phase when the COM is within the BoS, balance is challenged as the velocity of the COM is redirected laterally from the unloading to the loading leg(MacKinnon and Winter 1993; Winter 1995). Thus, dynamic balance is defined as the ability to avoid a fall while the COM is in a constant state of motion both within and outside of the BoS. In this scenario, balance is achieved by the neuromuscular system counteracting the gravitational and joint reaction forces acting on the sinusoidal trajectory of the upper body (head, arms, and trunk) to avoid falling. As the trunk is the largest segment in the upper body, which accounts for two-thirds of total-body mass, large variations in its rhythmic trajectory (hereby termed postural control) are closely linked with reduced dynamic balance (MacKinnon and Winter 1993).
One of the primary mechanisms to counteract these destabilizing forces and maintain balance is effective foot placement (Winter 1995; Bruijn and van Dieën 2018). To achieve appropriate and effective foot placement, the neuromuscular system predicts the future kinematic state of the COM to determine upcoming foot placement at initial-contact (Bruijn and van Dieën 2018). In the anteroposterior direction, the passive dynamics of the body are exploited requiring minimal guiding information from automated subcortical and brain stem regions to determine foot placement (Bruijn and van Dieën 2018; Siragy and Nantel 2018; Bauby and Kuo 2000). In contrast, effective mediolateral foot placement requires active sensory information processing from higher-level cortical regions (Bruijn and van Dieën 2018). Specifically, in this direction, the visual and vestibular systems provide information about head orientation which is then integrated with proprioceptive input from the trunk (Bruijn and van Dieën 2018). The culmination of this process provides the neuromuscular system with an estimate of the dynamical state (position, velocity, and acceleration) of the COM which it then utilizes to determine upcoming mediolateral foot placement (Bruijn and van Dieën 2018; Hurt et al. 2010). Indeed, previous research demonstrates that upcoming foot placement is determined by the kinematic state of the COM during the preceding contralateral mid-stance (Bruijn and van Dieën 2018; Hurt et al. 2010; Wang and Srinivasan 2014; Perry and Srinivasan 2017).
However, the use of HMD to project VR environments alters the visual input provided to the sensorimotor system from that of the real-world. This may create a sensory conflict when integrating the multiple inputs necessary for stable walking. Specifically, visual input is derived from the VR-HMD but the remaining sensory inputs (such as vestibular and proprioceptive) stem from the real-world. While there are several theories about sensory conflict the most recent theory was proposed by Palmisano et al. (2023) who showed that during HMD-based VR, display lag can results in differences between the virtual and physical head pose, a sensory conflict that can also be a primary trigger for cybersickness. Although overground VR gait studies have yet to quantify dynamic balance and postural control, emerging research demonstrates that when individuals walk while wearing a VR-HMD, there is a decrease in both stride length and gait speed (Martelli et al. 2019), an increase in double support time (Janeh et al. 2017), a decrease in cadence (Canessa et al. 2019), and an increase in step length, width, and time variability (Martelli et al. 2019; Yamagami et al. 2020; Horsak et al. 2021). The culmination of this evidence indicates that individuals adopt a “cautious gait” strategy when walking with a VR-HMD compared to the real-world (Horsak et al. 2021). This potentially suggests that when walking with a VR-HMD, dynamic balance may be threatened. However, the current gait kinematics and kinetics reported in the literature do not quantify stability while walking. This aspect is critical to examine as the use of a VR-HMD may increase the risk of falling while walking.
Therefore, the purposes of this study are to assess whether walking with VR-HMD affects (1) postural control, (2) dynamic balance, and (3) the relationship between the COM and foot placement compared to walking in the real-world. To this end, a secondary analysis was conducted from our recently published study that examined gait kinematic and kinetic changes during overground walking with a VR-HMD (Horsak et al. 2021). These results will serve as a guide for future researchers wishing to use VR-HMD to assess or train dynamic balance in fall-prone demographics.
2 Methods
2.1 Participants
A convenience sample of 9 males and 12 female healthy volunteers (N = 21, age: 37.6 ± 8.6 years, weight: 70.8 ± 14.9 kg, height: 169.6 ± 6.8 cm) was recruited at our University’s campus located in St. Pölten in Austria. We included healthy volunteers aged between 18 to 65 years and excluded participants presenting with any temporary or long-term conditions affecting their ability to walk or affecting their ability to navigate through the VR (e.g., visual impairments). This study was approved by the local ethics committee (GS1-EK-4/682-2020) and was performed in accordance with the relevant guidelines and regulations. All participants were informed prior to the study and gave written informed consent.
2.2 Procedures
This is a secondary analysis from our recently published study that examined gait kinematic and kinetic changes during overground walking with a VR-HMD (Horsak et al. 2021). All participants underwent standard 3D gait analysis in four randomly assigned walking conditions by computer-generated numbers: the real laboratory (RLab, size: 11.9 × 5.4 m), a virtual laboratory resembling the real world (VRLab), and two differently sized versions of the VRLab. The latter two were excluded for this study because we did not observe any additional findings between the normal VRLab condition and the two differently sized VRLab versions warranting their inclusion in the current analysis (Horsak et al. 2021). Participants wore the HMD for approximately 7 ± 1 min per VR condition and approximately 21 min in total during the entire study.
2.3 Virtual reality room-scale environment
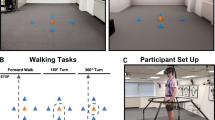
To fully immerse participants in a first-person view in the room-scale VR environment, they wore an HTC Vive Pro HMD which was operated wirelessly and calibrated to the real-world (Fig. 1). This allowed them to navigate and walk freely in the virtual environment. We have used the physical dimensions of the real laboratory to create a virtual 3D model of the laboratory and its interior. The Unity3D game engine was then utilized to visualize the laboratory. We developed a middleware service to combine data from multiple sensor input systems, such as a motion capture system and HTC Vive 2.0 trackers, and communicate with the VR application via the User Datagram Protocol (UDP). One HTC Vive 2.0 tracker was strapped to each foot to track and display the feet in VR in real-time. This allowed volunteers to have a visual indication of their current position in the VRLab and their body posture while navigating through the VR environment. Five HTC Vive Lighthouses (2.0) were used to continuously track the positions of the HMD and both trackers in the real-world. The HTC Vive Lighthouses did not interfere with the motion capture system.
The real-world laboratory (RLab) partially superimposed with the virtual environment (VRLab) the participants saw. Left: Participant walking in the laboratory immersed with a head-mounted-display and equipped with the skin-mounted retroreflective markers for motion capturing. HTC Vive 2.0 trackers were used to track the positions of the feet in real-time and display them in the VR. Right: View of the real laboratory and how it looked in the VR. Right image partly reused from Horsak et al. (2021)
2.4 3D gait analysis
A 12-camera motion capture system (Nexus, 2.11, Vicon, Oxford, UK) was used to collect trajectory data at 150 Hz while one synchronized force plate (Kistler, Winterthur, CH) was used for collecting ground reaction force data at 300 Hz. Participants walked at self-selected walking speed in all conditions up and down an approximately 7 m long walk way, where the force plate was embedded flush with the ground (see Fig. 1). To collect participants’ kinematics, the extended Cleveland Clinic marker set (Baker 2013) was used for the lower extremity and combined with the Vicon Plug-In-Gait model for the upper body. To determine the hip joint center, the regression equation from Davis et al. (1991) was used. Kinetic data were filtered using a 4th-order zero-lag Butterworth filter with a cut-off frequency of 20 Hz. All raw kinematic trajectories were filtered using the Vicon Nexus system integrated Woltring filtering routine with an MSA value of 15. For the analysis, all available steps including the ones before and after the force plate (except those close to the end of the walkway), were used. This resulted in 23 ± 6 steps per participant and body side.
2.5 Outcome parameters
Data were analyzed in custom Matlab (2022b) and Python 3.8 scripts (using Spyder 4.1.5 within the Anaconda 3.0 suite). Gait events were determined via the force plate with a threshold of 20 N and by using an auto-correlation function and manual inspection for all steps before and after the force plate. All data were time normalized and expressed as a percentage of the gait cycle. To mitigate the influence of gait direction on our outcome parameters, i.e. walking not completely parallel to the anteroposterior axis of the laboratory coordinate system, a similar approach to (Kainz et al. 2016) was chosen. Relevant trajectories for each stride were rotated by the angle determined between the gait direction and the anteroposterior direction of the laboratory for each stride separately. The rotation was then conducted about the pelvis center, defined as the mid-point between both hip joint center locations.
Dynamic balance and postural control were assessed with three outcomes: (1) the Margin of Stability (MOS) in the anteroposterior (AP-MOS) and mediolateral (ML-MOS) directions at initial-contact (Hof et al. 2007), (2) the relationship between the mediolateral COM position and acceleration at mid-stance with subsequent step width (Hurt et al. 2010), and (3) trunk kinematics during the entire gait cycle.
2.5.1 Margin of stability
The MOS was calculated bilaterally and defined as the distance of the extrapolated COM (xCOM) to the heel-marker for the AP-MOS or ML-MOS using Eqs. (1 and 2). See Fig. 2 for a schematic description.
where vCOM is the velocity of the COM, g is the gravitational acceleration and l is the height of the inverted pendulum which was determined as the distance of the right/left heel marker to the COM at initial-contact. The position of the COM was estimated by using the cumulative mass and position of each anthropometric segment based on the Cleveland Clinic model (Baker 2013) and Vicon’s Plug-In-Gait upper body model. Hof et al. (2007) originally proposed to use the center of pressure as the BoS. However, we included all steps during walking along the walkway including those without force plate contact, and therefore used the position of the heel-marker as an approximation of the COP during initial-contact. The MOS calculation is dependent on the foot and walking direction. To account for this, we have used the (− 1) term to correct for the directions of the BoS and xCOM vectors and ensure the MOS is positive when the xCOM is medial compared to the BoS or negative when the xCOM is anterior to the BoS. If the xCOM would be lateral to the BoS a person would have experienced a loss of balance. Note that a negative AP-MOS indicates that the xCOM is progressing forward, which is the goal of forward walking. Since velocity is taken into account in the xCOM, the AP-MOS will lie outside the BoS during initial contact (IC).
2.5.2 Relationship of COM state to step width
To assess the ability of the dynamical state of the trunk to determine mediolateral foot placement, we used similar to Hurt et al. (2010), a multiple linear regression model to relate the trunk COM position and its acceleration at mid-stance with the subsequent step width. Mid-stance was defined as the time point when the frontal velocity of the trunk COM was zero. The COM position was defined as the horizontal distance between the vertical projection of the COM on the ground and the mid-point of the line connecting the heel and toe markers of the stance foot at mid-stance. Step width was calculated as the frontal plane distance of the mid-point between toe- and heel-markers for both feet during two consecutive steps (see Fig. 2). For the regression analysis, the trunk COM positions and accelerations at mid-stance for each left and right step were paired with the following step widths for each participant and lumped together in one sample. This resulted in a regression equation as followed for each walking condition. The explained variance in terms of the coefficient of determination (\(R^2\)) served as the outcome.
2.5.3 Trunk kinematics
Lastly, to assess postural control we used the AP, ML, and vertical kinematics of the trunk to understand the impact of the VR on trunk kinematics. The T10-marker, placed right above the tenth thoracic vertebra, served as a surrogate to describe trunk motion. We have calculated the velocity profiles as well as the variability in terms of its standard deviation for each individual across the entire gait cycle and averaged them between the left and right steps to have robust and representative values per person.
Schematic depicting some of our outcome variables relevant for this study. Left image: the margin of stability (MOS) was calculated for the mediolateral (ML-MOS) and anteroposterior (AP-ML) directions as the distance between the base of support (BoS) represented by the heel-marker at initial contact and the extrapolated center of mass position (xCOM). Right image: regarding the multiple linear regression, the mediolateral center of mass (COM) position and its acceleration during mid-stance were used to predict the subsequent step width. The COM position was defined as the horizontal distance between the vertical projection of the COM on the ground and the mid-point of the line connecting the heel- and toe-markers of the stance foot at mid-stance
2.6 Statistical analysis
Statistical analysis was conducted using SPSS Statistics 27 (IBM Corporation, NY, USA). Basic features of the data are reported as means and one standard deviation (SD), unless otherwise stated. Assumption of normality was checked by using a Shapiro–Wilk test and by inspecting the histogram of each variable.
2.6.1 Margin of stability
As initial dependent pairwise tests did not indicate any significant asymmetries in MOS variables between the left and right side, values of both sides were averaged to have a single robust representation of each individual. Then, depending on the normality of the data either dependent t-tests or Wilcoxon signed rank tests were used to evaluate if a significant difference exists in the mean or median of the AP-MOS and ML-MOS for walking in RLab compared to walking in the VRLab. Due to the explorative character of this study and the small number of family-wise tests conducted we refrained from using Bonferroni-corrections to avoid high Type II errors. Alpha level was set to 0.05.
2.6.2 Relationship of COM state to step width
Regarding the assumptions for the regression analysis, a Durbin-Watson test was used to check for potential autocorrelation. Values in the range of 1–3 were interpreted as acceptable. Visual inspection of the histograms as well as skewness and kurtosis were used to test if residuals were approximately normal, the Goldfeld–Quandt test was used to test for homoscedasticity, and lastly the variance inflation factor (VIF) was used to gauge multicollinearity between predictors. Variance inflation factor values of greater than 2 were interpreted as a presence of moderate to strong multicollinearity.
2.6.3 Trunk kinematics
Lastly, we utilized statistical parameter mapping (SPM) and the SPM1D package (v.0.4.2) available for Python (Pataky 2012) to identify any differences in trunk kinematic velocity profiles and their variability between both walking conditions and separately for all three anatomical planes. For this purpose we used paired-sampled t-tests, or in case of none-normality of the data, non-parametric tests. Alpha level was set to 0.05.
3 Results
3.1 Margin of stability
A Shapiro–Wilk test indicated a normal distribution for the AP-MOS but a non-normal distribution for ML-MOS. A dependent t-test identified a significant difference in AP-MOS between walking in the RLab (− 179 ± 34 mm) compared to walking in the VRLab (− 158 ± 32 mm), t(20) =\(-\)4.991, p <.001. A Wilcoxon signed-rank test indicated no significant change for ML-MOS between walking in the RLab compared to the VRLab (Z = − .886, p =.375). Median ML-MOS was 19 mm (IQR: 11 mm) and 20 mm (IQR: 11 mm) for walking in RLab and VRLab, respectively. Results of the MOS are visualized in rain cloud box plots (Allen et al. 2021) in Fig. 3.
3.2 Relationship of COM state to step width
Regarding the multiple regression, both variables, mediolateral COM position and acceleration, significantly predicted step width and both variables added significantly to the prediction (p < 0.05) in both walking conditions. During walking in the RLab both variables accounted for 54% of the variance, F(2741) = 434.7, p <.001, \(R^2\) =.540. During walking in the VRLab, 64% of the variance was explained F(2812) = 717.4, p <.001, \(R^2\) =.639. Fig. 4 shows a visualization of the 3D planes created by the regression equation for both walking conditions as well as the equation itself. From a goodness-of-fit perspective both regression models seemed appropriate and showed similar features in quality criteria. Durbin-Watson statistics was approximately 1.1, indicating moderate but acceptable auto-correlation, residuals were approximately normally distributed, the VIF (< 1.5) did not indicate high multicollinearity, and the Goldfeld–Quandt test was not significant (p > 0.6) indicating homoscedasticity.
3.3 Trunk kinematics
SPM Shapiro-Wilk tests indicated non-normal distributions in some variables. Non-parametric paired sample SPM t-tests identified a highly significant decrease in absolute trunk velocity for the anteroposterior (\(-\)7.2 ± 0.3%, p < 0.001) and vertical (\(-\)8.3 ± 5.7%, p < 0.001) directions and a significantly increased mediolateral trunk velocity (5.5 ± 5.6%, p < 0.05) during early stance and early swing (p < 0.05) when walking in the VRLab compared to the RLab (see Fig. 5). In addition, SPM identified an increased variability in mediolateral trunk velocity (+ 25.3 ± 4.1%, p < 0.001) in the VRLab compared to RLab. No relevant changes in variability were found in the anteroposterior or vertical direction.
Mediolateral margin of stability (ML-MOS) and anteroposterior MOS (AP-MOS) at initial contact (IC) for walking in the real laboratory (RLab) compared to walking in the virtual laboratory resembling the real one (VRLab). The plot shows the data distribution (probability density function), the jittered raw data, the mean (red line), and a box plot showing quartiles where whiskers extend to the end of the data distribution except for outliers (diamonds) (color figure online)
Three perspectives of a 3D visualization of the multiple linear regression model with mediolateral (ML) COM position (mm) and acceleration (mm/\(s^2\)) as predictors for subsequent step width (mm). The upper row (pink) shows the model while walking in the real laboratory (RLab), the lower row (blue) depicts the model while walking in the virtual laboratory resembling the real one (VRLab) (color figure online)
Kinematic profiles of the trunk velocity (first row) and its variability (second row) for the anteroposterior, mediolateral, and inferior-superior direction when walking in the real and the virtual laboratory. The bar below each graph indicates when the SPM\(\{t\}\) test statistic exceeded the critical threshold. Grey bars indicate a p < 0.05, black bars a p < 0.001 (color figure online)
4 Discussion
This study analyzed the effect of walking in an overground VR-HMD on postural control, dynamic balance, and the coupling between the COM-BoS in healthy young adults. Our results demonstrated that walking in VR increases mediolateral trunk linear velocity variability, decreases AP-MOS, does not change the ML-MOS, and increases the relationship between the trunk’s dynamical state and subsequent step width compared to walking in the real-world. These findings are significant as instability and fall risk are greater in the mediolateral compared to the anteroposterior direction while walking (Winter 1995; Bruijn and van Dieën 2018; Siragy and Nantel 2018).
The increase in mediolateral trunk linear velocity variability may have stemmed from the difference in the visual scene between the VR and the real-world. Previous research demonstrates that mediolateral postural control while walking is heavily reliant on the effective processing of visual information to maintain stable motion (Bruijn and van Dieën 2018; Bauby and Kuo 2000; McAndrew et al. 2010, 2011; Osoba et al. 2019). Higher-level cortical structures integrate this input with somatosensory and vestibular input to provide information regarding the orientation and movement of the head relative to the trunk (Bruijn and van Dieën 2018; Bauby and Kuo 2000; Anson et al. 2014). In turn, this provides the neuromuscular system with an estimation of COM kinematics during walking (Bruijn and van Dieën 2018). In our study, the increased mediolateral trunk velocity variability may indicate that participants had difficulty in effectively integrating the visual input from the VR-HMD with the vestibular and proprioceptive input that was derived from the real-world. As such, a sensory conflict may have arisen which acted as a sensorimotor perturbation to destabilize upper body posture in this direction. To rule out that the observed increased variability is only caused by the reduced walking speed, as slower walking speed is associated with increased kinematic trunk variability (Dingwell and Marin 2006), we performed additional analysis and used a Pearson’s correlation to relate the change in walking speed (in terms of anteroposterior trunk velocity) and the increase in mediolateral variability of trunk velocity. No significant relationship was observed (see Fig. 6) which further strengthens the notion that the VR-HMD acted as a sensorimotor perturbation to our participants. While our study is the first to examine the effect of VR-HMD on postural control while walking, previous research has indicated that a sensory conflict, between the visual and vestibular systems, when using VR can induce postural instability during static standing (Chang et al. 2020; Akiduki et al. 2003). For instance, Imaizumi et al. (2020) evaluated the effect of wearing an HMD on standing postural control and found an increased body sway while wearing an HMD compared to not wearing one during their eyes open condition. They suggested that the HMD changed the visual-feedback-based postural control by possibly reducing visual information about the position and motion of the head with respect to participants’ surroundings (Imaizumi et al. 2020). Subsequently, this caused a faster, more variable, and more adjustable body sway when wearing the HMD during standing.
Interestingly, despite the increases in mediolateral trunk variability, no changes occurred in the ML-MOS. Indeed, as the trunk accounts for the majority of our total-body mass, increases in trunk kinematic variability would disrupt the regular and sinusoidal movement of the COM during the gait cycle which threatens dynamic balance (Siragy and Nantel 2018; Siragy et al. 2020). However, in our preceding study (Horsak et al. 2021) on this sample, we observed a simultaneous increase in participants’ step width variability. Although increased variability is a marker for falls in older adults (Verghese et al. 2009; Hausdorff et al. 2001), current evidence suggests that increases in spatiotemporal variability may also indicate foot placement adjustment to support a destabilized upper body in healthy young adults (Siragy et al. 2020; Siragy and Nantel 2018). Adjusting foot placement, as reflected by increased spatiotemporal variability, would account for the lack of findings in ML-MOS as participants modified their base of support to maintain their pre-existing level of dynamic balance in this direction (Siragy and Nantel 2018; Siragy et al. 2020; Rosenblatt and Grabiner 2010). Adjustment of foot placement to maintain dynamic balance would coincide with the observed ”cautious gait” strategy (slower walking speed, longer stride time, and increased double support time) implemented by our participants (Horsak et al. 2021). When dynamic balance is threatened, individuals take slower steps to provide additional time for the COM to transition from the unloading to the loading leg during double-support (Maki 1997; Herman et al. 2005). Further, the cautious gait strategy would account for the reduced AP-MOS observed during the VR condition compared to the real-world condition. Yang and King (2016) suggested that individuals implement this strategy to reduce the distance of the dynamic state of the COM to the BoS to reduce the likelihood of falling. As the dynamical state of the COM is located ahead of the BoS during steady-state walking, reducing the distance of the AP-MOS would facilitate the ability of individuals to return it within the BoS during a potential loss of balance (i.e. when encountering an external perturbation). Further, recently Alhirsan et al. (2023) have shown that there is a relationship between balance confidence and walking speed in virtual environments. In their study patients post stroke with high balance confidence walked faster in VR than patients with low confidence. Thus, in our study, participants may have adopted a slower walking speed and a cautious gait strategy to reduce the likelihood of a fall while wearing the VR-HMD.
The threat to dynamic balance, and the ensuing strategies to mitigate balance loss, would explain the increased R\(^2\) values during the VR condition. Indeed, we observed that COM kinematics (position and acceleration) accounted for 64% of the variance in step width during the VR condition compared to 54% during real-world walking. Hurt et al. (2010) proposed that a stronger relationship between upper body kinematics and step width may indicate increased voluntary control to maintain dynamic balance while walking. In our study, the increased mediolateral trunk velocity variability may have acted as an internal perturbation to our participant’s dynamic balance. Thus, in addition to the cautious gait strategy, our participants may have consciously tightened the coupling between their COM and foot placement to avoid a loss of balance. However, it is unclear whether the change in magnitude of 10%, compared to a relative change of either 15 or 20%, holds a direct implication for dynamic balance. Interestingly, Hurt et al. (2010) reported that older adults had an approximately 9% increase in this coupling compared to the younger adults in their study. This might provide an approximate indication as older adults have reduced dynamic balance compared to healthy young adults (Siragy and Nantel 2018). Hurt et al. (2010), further suggested this increase in older adults was indicative of a more active strategy to control gait. However, in their study, a treadmill was used to assess gait, and the extent to which the relationship between variations in step width and COM state differs between treadmill walking and overground is presently not known. Thus, future research should examine whether relative changes in magnitude affect an individual’s dynamic stability level as well as if treadmills further influence the relationship between step width and the COM state. Additionally, it is unclear whether and to what extent changes in walking speed affect the R\(^2\) values.
4.1 Limitations
Recent research indicates that VR-based exergaming can effectively increase balance and reduce the fear of falling in elderly individuals (Zahedian-Nasab et al. 2021; Mirelman et al. 2010; Lima Rebêlo et al. 2021). Virtual reality is also increasingly used in patients with neurological disorders, such as Parkinson’s Disease. A systematic review and meta-analysis (Wu et al. 2022) recently confirmed that VR-assisted balance training is highly effective in improving balance in patients with Parkinson’s Disease. While to date, the majority of research only uses non-immersive VR systems such as Microsoft’s Xbox and its wireless Kinect tracking system or non-immersive VR combined with treadmill training, the speed at which immersive VR technologies are currently developing suggests that there might be applications for fall-prevention and functional training in near future where highly immersive VR could play an important role. However, before such immersive VR technologies can serve as purposeful tools, we need to fully understand the impact they have on gait mechanics. Unfortunately, as our study sample of 21 participants is rather small and only comprised healthy individuals aged between 21 and 56 years, our results are limited in their generalizability to the elderly population or patients with neurological disorders. The study by Yamagami et al. (2020) is the only study we are aware of which evaluated the effect of VR on gait characteristics in patients with Parkinson’s Disease. They investigated whether Freezing-of-Gait (FoG) provoking VR environments exacerbate gait impairments associated with FoG compared to unobstructed VR and the physical laboratory. They found that walking speed was reduced and gait variability increased when people with Parkinson’s Disease walked overground in all VR environments. While both their and our results point in the same direction, there is still a need to better understand the impact of immersive VR on gait characteristics in various demographics. This is important in order to fully exploit the potential of this rapidly advancing technology as a supportive tool for research and clinical care. Further, as we did not measure sensory conflict directly in our study, it is unclear if and to what extent this had an effect on our participants’ sensorimotor control to maintain dynamic balance. Lastly, our results need to be interpreted with caution when being used to inform the planning or development of prolonged exercise sessions using immersive VR as we do not know if the observed effects cease over a prolonged use or are independent of usage time and experience with VR. This is an important question that needs further attention.
Linear Pearson‘s correlation between the change in mediolateral variability of trunk velocity (y-axis) and the change in anteroposterior trunk velocity (x-axis) when walking in the virtual laboratory (VRLab) compared to the real laboratory (RLab). Differences were calculated as mean velocity during the entire gait cycle as VRLab-RLab
5 Conclusion
Recent studies in general observed a consistent pattern of gait adjustments when walking in VR overground environments compared to walking in reality. Most frequently reported effects are reduced walking speed, increased gait variability, and step width which all point towards adjustments to a more cautious gait. Our study further underscores this idea and is the first to provide an explanation from the perspective of dynamic stability and postural control. Our results indicate that the VR delivered to the participants with an HMD, results in an altered optical flow, indicated by increased mediolateral trunk kinematic variability, which seems to be compensated by the participants by slightly reweighing sensorimotor input. Subsequently, participants consciously tightened the coupling between their COM and foot placement to maintain their already existing level of mediolateral dynamic stability. Although our results show some adjustments in dynamic stability and postural control, these should not be overestimated as we already showed that overall effects on the gait kinematic and kinetic patterns are rather small. Immersive VR is a rapidly developing technology and it is reasonable to assume that VR and HMDs will become even more immersive in the near future thereby further reducing the effects they currently have on gait stability. Our study should thus be repeated in the foreseeable future with updated hardware and with various patient groups to support its application as a purposeful tool in healthcare.
Data availability
The experimental data related to this study are available via the St. Pölten University of Applied Sciences online data repository: https://phaidra.fhstp.ac.at/o:5475.
References
Ahn S, Hwang S (2019) Virtual rehabilitation of upper extremity function and independence for stoke: a meta-analysis. J Exerc Rehabilit 15(3):358–369. https://doi.org/10.12965/jer.1938174.087
Akiduki H, Nishiike S, Watanabe H et al (2003) Visual-vestibular conflict induced by virtual reality in humans. Neurosci Lett 340(3):197–200. https://doi.org/10.1016/s0304-3940(03)00098-3
Alhirsan SM, Capó-Lugo CE, Hurt CP et al (2023) The immediate effects of different types of augmented feedback on fast walking speed performance and intrinsic motivation after stroke. Arch Rehabilit Res Clini Trans 5(2):100265. https://doi.org/10.1016/j.arrct.2023.100265
Allen M, Poggiali D, Whitaker K et al (2021) Raincloud plots: a multi-platform tool for robust data visualization. Welcome Open Res 4:63. https://doi.org/10.12688/wellcomeopenres.15191.2
Anson E, Agada P, Kiemel T et al (2014) Visual control of trunk translation and orientation during locomotion. Exp Brain Res 232(6):1941–1951. https://doi.org/10.1007/s00221-014-3885-1
Baker R (2013) Measuring walking: a handbook of clinical gait analysis. Mac Keith Press, London
Bauby CE, Kuo AD (2000) Active control of lateral balance in human walking. J Biomech 33(11):1433–1440. https://doi.org/10.1016/S0021-9290(00)00101-9
Benham S, Kang M, Grampurohit N (2019) Immersive virtual reality for the management of pain in community-dwelling older adults. OTJR Occup Particip Health 39(2):90–96. https://doi.org/10.1177/1539449218817291
Brepohl PCA, Leite H (2023) Virtual reality applied to physiotherapy: a review of current knowledge. Virtual Real 27(1):71–95. https://doi.org/10.1007/s10055-022-00654-2
Bruijn SM, van Dieën JH (2018) Control of human gait stability through foot placement. J R Soc Interface 15(143):20170816. https://doi.org/10.1098/rsif.2017.0816
Canessa A, Casu P, Solari F, et al (2019) Comparing real walking in immersive virtual reality and in physical world using gait analysis. In: Proceedings of the 14th international joint conference on computer vision, imaging and computer graphics theory and applications. SCITEPRESS—Science and Technology Publications, Prague, Czech Republic, pp 121–128
Canning CG, Allen NE, Nackaerts E et al (2020) Virtual reality in research and rehabilitation of gait and balance in Parkinson disease. Nat Rev Neurol 16(8):409–425
Chang E, Kim HT, Yoo B (2020) Virtual reality sickness: a review of causes and measurements. Int J Hum Comput Interact 36(17):1658–1682. https://doi.org/10.1080/10447318.2020.1778351
Chen B, Liang RQ, Chen RY et al (2021) The effect of virtual reality training on the daily participation of patients: a meta-analysis. Complement Ther Med 58:102676. https://doi.org/10.1016/j.ctim.2021.102676
Darter BJ, Wilken JM (2011) Gait training with virtual reality-based real-time feedback: improving gait performance following transfemoral amputation. Phys Ther 91(9):1385–1394. https://doi.org/10.2522/ptj.20100360
Davis RB, Õunpuu S, Tyburski D et al (1991) A gait analysis data collection and reduction technique. Hum Mov Sci 10(5):575–587. https://doi.org/10.1016/0167-9457(91)90046-Z
de Oliveira JM, Fernandes RCG, Pinto CS et al (2016) Novel virtual environment for alternative treatment of children with cerebral palsy. Comput Intell Neurosci 2016:e8984379. https://doi.org/10.1155/2016/8984379
de Vries AW, Willaert J, Jonkers I et al (2020) Virtual reality balance games provide little muscular challenge to prevent muscle weakness in healthy older adults. Games Health J 9(3):227–236
Delgado F, Der Ananian C (2021) The use of virtual reality through head-mounted display on balance and gait in older adults: a scoping review. Games Health J 10(1):2–12. https://doi.org/10.1089/g4h.2019.0159
Dingwell JB, Marin LC (2006) Kinematic variability and local dynamic stability of upper body motions when walking at different speeds. J Biomech 39(3):444–452. https://doi.org/10.1016/j.jbiomech.2004.12.014
Hausdorff JM, Rios DA, Edelberg HK (2001) Gait variability and fall risk in community-living older adults: a 1-year prospective study. Arch Phys Med Rehabil 82(8):1050–1056. https://doi.org/10.1053/apmr.2001.24893
Herman T, Giladi N, Gurevich T et al (2005) Gait instability and fractal dynamics of older adults with a“cautious’’gait: Why do certain older adults walk fearfully? Gait Posture 21(2):178–185. https://doi.org/10.1016/j.gaitpost.2004.01.014
Hof AL, van Bockel RM, Schoppen T et al (2007) Control of lateral balance in walking. Gait Posture 25(2):250–258. https://doi.org/10.1016/j.gaitpost.2006.04.013
Horsak B, Simonlehner M, Schöffer L et al (2021) Overground walking in a fully immersive virtual reality: a comprehensive study on the effects on full-body walking biomechanics. Front Bioeng Biotechnol 9:1236. https://doi.org/10.3389/fbioe.2021.780314
Hurt CP, Rosenblatt N, Crenshaw JR et al (2010) Variation in trunk kinematics influences variation in step width during treadmill walking by older and younger adults. Gait Posture 31(4):461–464
Imaizumi LFI, Polastri PF, Penedo T et al (2020) Virtual reality head-mounted goggles increase the body sway of young adults during standing posture. Neurosci Lett 737:135333. https://doi.org/10.1016/j.neulet.2020.135333
Janeh O, Steinicke F (2021) A review of the potential of virtual walking techniques for gait rehabilitation. Front Hum Neurosci 15:646
Janeh O, Langbehn E, Steinicke F et al (2017) Walking in virtual reality: effects of manipulated visual self-motion on walking biomechanics. ACM Trans Appl Percept 14(2):1–15. https://doi.org/10.1145/3022731
Kainz H, Lloyd DG, Walsh HPJ et al (2016) Instantaneous progression reference frame for calculating pelvis rotations: reliable and anatomically-meaningful results independent of the direction of movement. Gait Posture 46:30–34. https://doi.org/10.1016/j.gaitpost.2016.02.011
Lee K (2020) Virtual reality gait training to promote balance and gait among older people: a randomized clinical trial. Geriatrics 6(1):1
Lima Rebêlo F, de Souza Silva LF, Doná F et al (2021) Immersive virtual reality is effective in the rehabilitation of older adults with balance disorders: a randomized clinical trial. Exp Gerontol 149:111308. https://doi.org/10.1016/j.exger.2021.111308
Lu Y, Ge Y, Chen W et al (2021) The effectiveness of virtual reality for rehabilitation of Parkinson disease: an overview of systematic reviews and meta-analyses. Syst Rev 11(1):1–14. https://doi.org/10.21203/rs.3.rs-255702/v1
MacKinnon CD, Winter DA (1993) Control of whole body balance in the frontal plane during human walking. J Biomech 26(6):633–644. https://doi.org/10.1016/0021-9290(93)90027-C
Maki BE (1997) Gait changes in older adults: predictors of falls or indicators of fear? J Am Geriatr Soc 45(3):313–320. https://doi.org/10.1111/j.1532-5415.1997.tb00946.x
Martelli D, Xia B, Prado A et al (2019) Gait adaptations during overground walking and multidirectional oscillations of the visual field in a virtual reality headset. Gait Posture 67:251–256
McAndrew PM, Dingwell JB, Wilken JM (2010) Walking variability during continuous pseudo-random oscillations of the support surface and visual field. J Biomech 43(8):1470–1475. https://doi.org/10.1016/j.jbiomech.2010.02.003
McAndrew PM, Wilken JM, Dingwell JB (2011) Dynamic stability of human walking in visually and mechanically destabilizing environments. J Biomech 44(4):644–649. https://doi.org/10.1016/j.jbiomech.2010.11.007
Mirelman A, Patritti BL, Bonato P et al (2010) Effects of virtual reality training on gait biomechanics of individuals post-stroke. Gait Posture 31(4):433–437
Osoba MY, Rao AK, Agrawal SK et al (2019) Balance and gait in the elderly: a contemporary review. Laryngoscope Investig Otolaryngol 4(1):143–153. https://doi.org/10.1002/lio2.252
Palacios-Navarro G, Hogan N (2021) Head-mounted display-based therapies for adults post-stroke: a systematic review and meta-analysis. Sensors 21(4):1111. https://doi.org/10.3390/s21041111
Palmisano S, Allison RS, Teixeira J et al (2023) Differences in virtual and physical head orientation predict sickness during active head-mounted display-based virtual reality. Virtual Real 27(2):1293–1313. https://doi.org/10.1007/s10055-022-00732-5
Pataky TC (2012) One-dimensional statistical parametric mapping in Python. Comput Methods Biomech Biomed Engin 15(3):295–301. https://doi.org/10.1080/10255842.2010.527837
Peng QC, Yin L, Cao Y (2021) Effectiveness of virtual reality in the rehabilitation of motor function of patients with subacute stroke: a meta-analysis. Front Neurol 12:639535. https://doi.org/10.3389/fneur.2021.639535
Perry JA, Srinivasan M (2017) Walking with wider steps changes foot placement control, increases kinematic variability and does not improve linear stability. R Soc Open Sci 4(9):160627. https://doi.org/10.1098/rsos.160627
Rosenblatt NJ, Grabiner MD (2010) Measures of frontal plane stability during treadmill and overground walking. Gait Posture 31(3):380–384. https://doi.org/10.1016/j.gaitpost.2010.01.002
Siragy T, Nantel J (2018) Quantifying dynamic balance in young, elderly and parkinson’s individuals: a systematic review. Front Aging Neurosci 10:387
Siragy T, Mezher C, Hill A et al (2020) Active arm swing and asymmetric walking leads to increased variability in trunk kinematics in young adults. J Biomech 99:109529. https://doi.org/10.1016/j.jbiomech.2019.109529
Statista (2021) Extended reality (XR): AR, VR, and MR. http://www.statista.com/study/71389/extended-reality-xr/
Triegaardt J, Han TS, Sada C et al (2020) The role of virtual reality on outcomes in rehabilitation of Parkinson’s disease: Meta-analysis and systematic review in 1031 participants. Neurol Sci Off J Ital Neurol Soc Ital Soc Clini Neurophysiol 41(3):529–536. https://doi.org/10.1007/s10072-019-04144-3
Verghese J, Holtzer R, Lipton RB et al (2009) Quantitative gait markers and incident fall risk in older adults. J Gerontol Ser A 64A(8):896–901. https://doi.org/10.1093/gerona/glp033
Wang Y, Srinivasan M (2014) Stepping in the direction of the fall: the next foot placement can be predicted from current upper body state in steady-state walking. Biol Let 10(9):20140405. https://doi.org/10.1098/rsbl.2014.0405
Willaert J, De Vries AW, Tavernier J et al (2020) Does a novel exergame challenge balance and activate muscles more than existing off-the-shelf exergames? J Neuroeng Rehabil 17(1):6. https://doi.org/10.1186/s12984-019-0628-3
Winter D (1995) Human balance and posture control during standing and walking. Gait Posture 3(4):193–214. https://doi.org/10.1016/0966-6362(96)82849-9
Wu J, Zhang H, Chen Z et al (2022) Benefits of virtual reality balance training for patients with Parkinson disease: systematic review, meta-analysis, and meta-regression of a randomized controlled trial. JMIR Ser Games 10(1):e30882. https://doi.org/10.2196/30882
Yamagami M, Imsdahl S, Lindgren K et al (2020) Effects of virtual reality environments on overground walking in people with Parkinson disease and freezing of gait. Disabil Rehabil Assist Technol 18(3):266–273
Yang F, King GA (2016) Dynamic gait stability of treadmill versus overground walking in young adults. J Electromyogr Kinesiol 31:81–87
Zahedian-Nasab N, Jaberi A, Shirazi F et al (2021) Effect of virtual reality exercises on balance and fall in elderly people with fall risk: a randomized controlled trial. BMC Geriatr 21(1):509. https://doi.org/10.1186/s12877-021-02462-w
Acknowledgements
We would like to thank Lukas Richter for his thoughts and comments regarding the multiple linear regression analysis.
Funding
Open access funding provided by FH St. Pölten - University of Applied Sciences. This work received funding from the Austrian Research Promotion Agency (FFG) and the BMDW within the COIN-program (#866855) and from the Research Promotion Agency of Lower Austria (Gesellschaft für Forschungsförderung NÖ) within the Endowed Professorship for Applied Biomechanics and Rehabilitation Research (SP19-004).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
All participants were informed prior to the study and gave written informed consent.
Informed consent
All participants were informed prior to the study and gave written informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Horsak, B., Simonlehner, M., Dumphart, B. et al. Overground walking while using a virtual reality head mounted display increases variability in trunk kinematics and reduces dynamic balance in young adults. Virtual Reality 27, 3021–3032 (2023). https://doi.org/10.1007/s10055-023-00851-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10055-023-00851-7