Abstract
End-stage liver failure is a condition of collapsing liver function with mortality rates up to 80. Liver transplantation is the only lifesaving therapy. There is an unmet need for therapy to extend the waiting time for liver transplantation or regeneration of the native liver. Here we review the state-of-the-art of non-cell based and cell-based artificial liver support systems, cell transplantation and plasma exchange, with the first therapy relying on detoxification, while the others aim to correct also other failing liver functions and/or modulate the immune response. Meta-analyses on the effect of non-cell based systems show contradictory outcomes for different types of albumin purification devices. For bioartificial livers proof of concept has been shown in animals with liver failure. However, large clinical trials with two different systems did not show a survival benefit. Two clinical trials with plasma exchange and one with transplantation of mesenchymal stem cells showed positive outcomes on survival. Detoxification therapies lack adequacy for most patients. Correction of additional liver functions, and also modulation of the immune system hold promise for future therapy of liver failure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
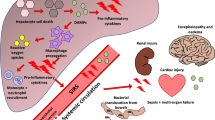
End-stage liver failure (ESLF) is a life-threatening condition of patients with collapsing liver function, caused by massive death of liver cells. The clinical syndrome comprises bleeding risks or thrombosis, disturbed acid–base homeostasis, systemic inflammatory response, hemodynamic instability, hepatic encephalopathy (HE) with the risk of increased intracranial pressure (ICP) and multi-organ failure.
Different types of ESLF are distinguished: acute liver failure (ALF), when ESLF occurs in a person with a previous healthy liver and Acute on Chronic Liver Failure (ACLF) in a patient with an already compromised liver, mostly cirrhosis.
The incidence of ACLF in the Western World is about 70.000 patients per year and for ALF about 8000 [1]. At present, standard medical therapy consists of treating the cause of deterioration, maintaining hemodynamic stability, fluid-, acid/base- and electrolyte balance, supplying fresh frozen plasma in case of bleeding, preventing increasing ICP and, optionally hemodialysis [2].
Nevertheless, mortality rates are high, up to 80%, depending on the cause of ESLF and the number of failing organs [3]. The heterogeneity between the pathophysiology of the ESLF patients severely complicates the standardization of an effective treatment [4]. At present liver transplantation (LTX) is the only lifesaving therapy. In the EU one-year survival rates after liver LTX are 74% for ALF patients and 85% for ACLF patients (European Liver Transplant Registry 1988–2015), however, the low supply of donor livers limits the impact of LTX [5].
There is an unmet need for improving standard medical therapy to such extent that the waiting time for LTX can be prolonged and the patient enters surgery in a better condition or, ideally, that the native liver regenerates.
Different liver support strategies have been developed, including non-cell based and cell-based artificial liver supportive systems (ALSS), cell transplantation and high volume plasma exchange.
Here, we summarize the state-of-the-art of liver support strategies for ESLF and analyze remaining problems and possible solutions.
Non-cell-based ALSS
All the non-cell-based ALSS rely on extracorporeal albumin purification, either by albumin dialysis, fractionated plasma separation, or replacement of albumin and/or adsorption techniques. These therapies aim to remove albumin-bound toxins which accumulate in the plasma. A limited number of studies has been performed on pigs with ALF caused by complete liver ischemia or overdose of acetaminophen (APAP). No ACLF models were tested. These experiments show that non-cell based albumin purification devices have the potential to improve biochemical parameters and ICP, while ADVOS and DIALIVE also improve survival time (Table 1).
Table 2 shows the results of clinical studies of non-cell based ALSS: two randomized clinical trials (RCTs), one controlled clinical trial (CCT) and one uncontrolled trial, as well as seven retrospective studies comparing two or more groups. Most of the studied treatments positively affected biochemical parameters and secondary endpoints, like HE, but whether a significant effect on the primary endpoint, i.e. improved survival rates, has been established, remains controversial [6]. A meta-analysis in 2013 of eight RCTs showed that non-cell based ALSS reduced mortality in ACLF patients (p < 0.018), but not in ALF patients [7]. In contrast, a meta-analysis in 2015 [8], comparing MARS treatment with standard medical therapy, showed significant effect on survival in 93 ALF patients (p = 0.04), and no survival effect in 453 ACLF patients. Subsequent clinical studies continued to produce contradictory results with albumin dialysis systems. Gerth et al. [9], in a retrospective study of 101 ACLF patients, confirmed improved short-term mortality in ACLF by MARS, but the same group failed to improve 28-day mortality in ALF [10]. These apparent contradictory results need further clarification. It is most likely that only specific subgroups of ESLF patients, i.e. those with less severe liver failure, may profit from non-cell based ALSS. A combination with plasma exchange (PE) seems to improve the impact of non-cell based ALSS therapy; controlled studies on non-cell based ALSS showed predominantly improved survival in those combination therapies (2 out of 2 studies [11, 12]), while stand-alone non-cell based ALSS therapies predominantly failed to provide any survival benefit (negative studies: [10, 13,14,15]) with the exception of two positive studies [9, 16].
In summary, different albumin purification devices unequivocally reduce elevated plasma bilirubin, and haemodialysis reduces plasma ammonia levels. Consequently, HE grade might be improved. This reduction of HE, may, in combination with removal of albumin-bound toxins ameliorate ESLF. According to the meta-analyses [7, 8] in a minority of cases, non-cell based ALSS improved survival time, but it is unlikely that stand-alone non-cell based ALSS will prevent liver transplantation, as normalization of coagulation, electrolyte balance, body homeostasis and cardiovascular stability requires a more complete restoration of failing liver function. Nevertheless, new systems, like DIALIVE and ADVOS, which show short-term survival benefit in animals with ALF, are under investigation, but we have to wait for their clinical benefit on survival.
Cell-based ALSS
Bioartificial liver (BAL) devices
A BAL is an extracorporeal device containing liver cells to be connected temporarily to the patient’s circulation compensating the failing detoxification, synthetic and homeostatic function of the diseased liver. An optimal BAL device promotes liver cell differentiation by allowing medium perfusion, 3-dimensional growth and supplying oxygen and contains sufficient liver cell mass (at least 20% of the native liver, approximating 15.109 or 150 g hepatocytes) that provides stable support of multiple liver-specific functions [17]. Therefore, a BAL has more potency to be effective than non-cell ALSS. Besides highly functional, the ideal biocomponent of BALs should be of human origin, safe and cost effective.
Many BAL-devices have been studied in experimental models of large animals with ALF (Table 3); only few have reached clinical application (Table 4).
Preclinical BAL studies
The applied large animal models relied on ALF caused by complete liver ischemia, total hepatectomy, overdose of drugs, such as D-galactosamine (D-GALN) and APAP, or intoxication with α-amanitin and lipopolysaccharide. Most controlled studies (CSs) included three groups; control with no connection to the system (c), empty-BAL (eB) and cell-filled-BAL (cB). Studies that did not include the eB group are less informative, as dilution of plasma or blood by pre-filling the extracorporeal circuit may lead to attenuation of ESLF. Most CSs in animals (13 out of 15) showed a beneficial effect on survival and biochemical markers. One study even reported that BAL treatment accelerated liver regeneration (56). So far, BAL systems have not been studied in animal models of ACLF.
Clinical BAL studies
Four different BAL systems based on freshly isolated porcine liver cells (BLSS, MELS, AMC-BAL and TECA-BALSS/HBAL) were tested in phase I/IIa studies and one, the HepatAssist, with cryopreserved porcine liver cells, in an RCT. All studies showed the safety of the system. However, the RCT with the HepatAssist device could not establish survival benefit in the whole patient population, analysed by intention to treat. Unexplained was an increased survival in the subpopulation of fulminant/subfulminant hepatic failure patients [18]. In the uncontrolled study of the AMC-BAL plasma levels of total bilirubin and ammonia decreased by 35 and 45%, respectively, and was associated with improved neurological state and stabilization of hemodynamics [19].
The ELAD system, based on the human liver cell line C3A, has been extensively tested in clinical trials with the largest ones being NCT01471028, an open-label RCT in 203 patients with severe alcoholic hepatitis [20], and very recently VTL-308 in 151 less severe and relatively young alcoholic hepatitis patients (age > 18 and < 50 years, MELD (Model for End-Stage Liver Disease) score < 30). Both RCTs showed temporary improvement of some biochemical parameters in ELAD-treated groups vs control groups, but no significant benefit on survival. By intent to treat analysis, ELAD did not meet its primary endpoint, overall survival (https://vitaltherapies.com/research-clinical-trials-development/elad-rostock-9-29-18/).
Concluding remarks clinically applied cell-based ALSS
Most BAL systems showed safety and efficacy in animal models of ESLF, indicating that supply of a broad spectrum of liver functions has a high potential to support ESLF patients. However, a clinical breakthrough has not yet been obtained, due to different factors. In Europe progress has been delayed due to the European moratorium on xenotransplantation [21]. The major concerns around BAL applications with xenogeneic liver cells included immunological responses and risks associated with zoonotic infections. So far, transmission of porcine endogenous retroviruses to patients treated with BAL containing porcine cells has not been observed [22, 23]. Consequently, outside Europe researchers continue developing BALs based on porcine hepatocytes; the SR-BAL and LifeLiver systems are pre-clinically under investigation (Table 3).
As an alternative for primary porcine hepatocytes, human liver cell lines have been most extensively applied as biocomponent for BALs. Their human origin, high reproducibility and relatively cheap propagation make them more suitable for BAL application. However, the cell lines most frequently used until now, C3A and HepG2, display a limited spectrum of liver functions and even produce lactate and ammonia, instead of eliminating it [17]. This may be a reason for the disappointing outcomes of the clinical ELAD trials. Another cell source comprises original or induced stem cells, which, however, display a limited functional spectrum as well [17, 24]. To date, the most functional human liver cell line is the HepaRG cell line [25], which further differentiates by culturing in the AMC-BAL system [26]. Comparison of the transcriptomes of different proliferative sources of human liver cells showed that HepaRG cells most closely resembled primary human hepatocytes [24, 27]. Further improvement of existing cell systems may be achieved by (conditional) ectopic expression of limiting regulatory or structural genes and by application of cell culture systems promoting maturation, e.g. by delivering high oxygen levels, 3D configuration and perfusion [28].
The development of BAL technology also faces a logistical challenge, as cells need to be provided with preservation of high functionality at the bedside. Cryopreservation of primary hepatocytes, however, may further aggravate the damage already induced by isolation [29], leading to cell death or dedifferentiation. This may be the reason for the disappointing results of the HepatAssist RCT [18]. Cryopreservation is substantially less damaging for proliferative cells than mature cells. In addition, liver cells rapidly deteriorate in the presence of human plasma, which limits the therapeutic window of the BAL [30, 31]. This negative effect can be reversed by an intermittent phase of recirculating culture medium through the bioreactor [32]. Another challenge that extracorporeal BALs face is the provision of in vivo like bile secretion [33]. A hybrid BAL containing a sufficient amount of well-functioning liver cells combined with integrated haemodialysis or albumin purification might be the preferred option to improve the patient’s condition to create time for native liver regeneration or liver transplantation. It is evident that for clinical application, the cell- and BAL-cultures need to be produced according to Good Manufacturing Practice [34].
Together, these challenges render a BAL system a complex product, and the costs of a BAL treatment will be substantial. This is actually a general problem for advanced therapies, based on cell or tissue-engineered products.
Other therapies
High volume plasma exchange
By plasma exchange combined with hemoperfusion or continuous hemodiafiltration overabundant toxic substances will be removed and reduced liver-specific products will be replenished. To our knowledge, no preclinical survival studies in large animals have been performed. The first large prospective CS in HBV-associated ACLF patients (MELD score 28–29) showed higher short- and long-term survival in the treatment group (n = 104) vs the control group (n = 130) [35].
In 2016 Larsen et al. [36] showed in a RCT that high volume plasma exchange (HVPE), with 8–12 L daily volume exchange, improved survival in patients with ALF (mainly APAP overdosed) by correcting the hemodynamics and biochemistry, e.g. the ammonia levels, and by modulating the innate and adaptive immune responses to the necrotic liver [37]. These two studies indicate a beneficial effect of PE or HVPE on ESLF patients. Further studies are needed to consolidate these results.
Hepatocyte or stem cell transplantation
Based on several successful survival studies in large animals [38,39,40], cell transplantation has been studied as an alternative way of filling the treatment gap at the ICU for ESLF patients [41].
Initially, hepatocytes were the cell source of choice for cell transplantation of ESLF patients. Five patients with ALF underwent intrasplenic or/and intrahepatic hepatocyte transplantation. All patients showed temporary clinical and biochemical improvement but eventually died [42]. A severe complication is that for treatment of ESLF large amounts of successfully engrafting and safe hepatocytes are needed. Therefore, hepatocyte transplantation is a more promising strategy for correcting liver-based metabolic deficiencies [43], requiring lower amounts of engrafted cells, and is less suitable for ESLF therapy.
Besides hepatocytes, mesenchymal stem cells (MSCs) have also been applied in cell transplantations in preclinical survival studies in large animals [44,45,46,47,48,49]. These cells do not directly support liver functions, but rather produce paracrine factors (e.g. cytokines, chemokines, and growth factors) with immunomodulatory and liver regeneration promoting effects [50].
A controlled study in HBV-associated ACLF patients compared the outcomes of four groups: controls (n = 30): PE (n = 30), umbilical cord-MSC transplantation (n = 30) and UC-MSC + PE (n = 20) [51]. It was concluded that MSC transplantation combined with PE treatment was safe, but could not significantly improve the short-term prognosis of HBV-ACLF patients compared to the single treatment.
An RCT on HBV-related ACLF patients showed that four weekly infusions with 100,000–1 million MSCs improved the MELD score and bilirubin levels, decreased the incidence of severe infections and increased the 24-week survival [52]. These studies indicate promising results. However, further studies are needed to show the benefit of MSC transplantation in all categories of ESLF patients.
Concluding remarks
Interesting developments are ongoing in the field of liver support for ESLF patients. From the first non-cell-based ALSS studies we learned that detoxification modalities may temporarily yield improvement of biochemistry and grade of HE, but the supply of plasma factors, control of homeostasis, and/or modulation of the immune system are needed to effectively support ESLF patients.
BAL systems were able to improve survival in experimental animal models of ALF, but due to practical and financial problems and usage of cells with low functionality, the clinical development remains behind. Other systems as PE and MSC transplantation, both modulating the immune responses, with PE also supplying detoxification and plasma proteins, hold promise for the future as well, as survival benefit has been shown in clinical trials.
The question rises which therapy should be used in which patients and at which stage of the disease? Table 5 compares advantages and disadvantages of different treatment modalities for ESLF patients. Apart from providing survival benefit also other factors are relevant for decision making, including the complexity, risks and costs of the procedure, and the status of the patient. For the future, we need improved metabolic and immunologic monitoring of ESLF patients in combination with detailed measurement of the effect of the different therapies. Subsequently, therapies can be selected on the basis of informative biomarkers. This will progress the care of ESLF patients towards a more patient-tailored approach, optionally by combining different treatment modalities at different stages of the disease.
References
Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–128.
Trovato FM, Rabinowich L, McPhail MJW. Update on the management of acute liver failure. Curr Opini Crit Care. 2019;25:157–64.
Samuel D, Coilly A. Management of patients with liver diseases on the waiting list for transplantation: a major impact to the success of liver transplantation. BMC Medicine. 2018;16:113.
EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J Hepatol. 2017;66:1047–81.
Northup PG, Intagliata NM, Shah NL, Pelletier SJ, Berg CL, Argo CK. Excess mortality on the liver transplant waiting list: unintended policy consequences and model for end-stage liver disease (MELD) inflation. Hepatol. 2015;61:285–91.
Stutchfield BM, Simpson K, Wigmore SJ. Systematic review and meta-analysis of survival following extracorporeal liver support. Br J Surg. 2011;98:623–31.
Zheng Z, Li X, Li Z, Ma X. Artificial and bioartificial liver support systems for acute and acute-on-chronic hepatic failure: a meta-analysis and meta-regression. Exp Ther Med. 2013;6:929–36.
He GL, Feng L, Duan CY, Hu X, Zhou CJ, Cheng Y, et al. Meta-analysis of survival with the molecular adsorbent recirculating system for liver failure. Int J Clin Exp Med. 2015;8:17046–54.
Gerth HU, Pohlen M, Tholking G, Pavenstadt H, Brand M, Husing-Kabar A, et al. Molecular adsorbent recirculating system can reduce short-term mortality among patients with acute-on-chronic liver failure-a retrospective analysis. Crit Care Med. 2017;45:1616–24.
Gerth HU, Pohlen M, Tholking G, Pavenstadt H, Brand M, Wilms C, et al. Molecular adsorbent recirculating system (MARS) in acute liver injury and graft dysfunction: results from a case-control study. PLoS ONE. 2017;12:e0175529.
Du WB, Li LJ, Huang JR, Yang Q, Liu XL, Li J, et al. Effects of artificial liver support system on patients with acute or chronic liver failure. Transplant Proc. 2005;37:4359–64.
Yao J, Li S, Zhou L, Luo L, Yuan L, Duan Z, et al. Therapeutic effect of double plasma molecular adsorption system and sequential half-dose plasma exchange in patients with HBV-related acute-on-chronic liver failure. J Clin Apher. 2019. https://doi.org/10.1002/jca.21690.
Kribben A, Gerken G, Haag S, Herget-Rosenthal S, Treichel U, Betz C, et al. Effects of fractionated plasma separation and adsorption on survival in patients with acute-on-chronic liver failure. Gastroenterol. 2012;142:782–9.
Banares R, Nevens F, Larsen FS, Jalan R, Albillos A, Dollinger M, et al. Extracorporeal albumin dialysis with the molecular adsorbent recirculating system in acute-on-chronic liver failure: the RELIEF trial. Hepatol. 2013;57:1153–62.
Piechota M, Piechota A, Misztal M, Bernas S, Pietraszek-Grzywaczewska I. An evaluation of the usefulness of extracorporeal liver support techniques in patients with severe liver dysfunction. Arch Med Sci. 2019;15:99–112.
Zhao WX, Liu XM, Yu CM, Xu H, Dai JR, Chen HY, et al. Peritoneal dialysis effectively removes toxic substances and improves liver functions of liver failure patients. Eur Rev Med Pharmacol Sci. 2018;22:2432–8.
Van Wenum M, Chamuleau R, van Gulik T, Siliakus A, Seppen J, Hoekstra R. Bioartificial livers in vitro and in vivo: tailoring biocomponents to the expanding variety of applications. Expert Opin Biol Ther. 2014;14:1745–60.
Demetriou AA, Brown RS, Busuttil RW, Fair J, McGuire BM, Rosenthal P, et al. Prospective, randomized, multicenter, controlled trial of a bioartificial liver in treating acute liver failure. Ann Surg. 2004;239:660–70.
van de Kerkhove MP, Di Florio E, Scuderi V, Mancini A, Belli A, Bracco A, et al. Phase I clinical trial with the AMC-bioartificial liver. Int J Artif Organs. 2002;25:950–9.
Thompson J, Jones N, Al-Khafaji A, Malik S, Reich D, Munoz S, et al. Extracorporeal cellular therapy (ELAD) in severe alcoholic hepatitis—a multinational, prospective, controlled. Random Trial Liver Transpl. 2018;24:380–93.
de Bousingen D. Europe supports moratorium on xenotransplantation. Lancet. 1999;353:476.
Di Nicuolo G, D'Alessandro A, Andria B, Scuderi V, Scognamiglio M, Tammaro A, et al. Long-term absence of porcine endogenous retrovirus infection in chronically immunosuppressed patients after treatment with the porcine cell-based Academic Medical Center bioartificial liver. Xenotransplant. 2010;17:431–9.
Sauer IM, Kardassis D, Zeillinger K, Pascher A, Gruenwald A, Pless G, et al. Clinical extracorporeal hybrid liver support—phase I study with primary porcine liver cells. Xenotransplant. 2003;10:460–9.
Gao X, Liu Y. A transcriptomic study suggesting human iPSC-derived hepatocytes potentially offer a better in vitro model of hepatotoxicity than most hepatoma cell lines. Cell Biol Toxicol. 2017;33:407–21.
Gripon P, Rumin S, Urban S, Le Seyec J, Glaise D, Cannie I, et al. Infection of a human hepatoma cell line by hepatitis B virus. Proc Natl Acad Sci U S A. 2002;99:15655–600.
Nibourg GA, Hoekstra R, van der Hoeven TV, Ackermans MT, Hakvoort TB, van Gulik TM, et al. Increased hepatic functionality of the human hepatoma cell line HepaRG cultured in the AMC bioreactor. Int J Biochem Cell Biol. 2013;45:1860–8.
Kvist AJ, Kanebratt KP, Walentinsson A, Palmgren H, O'Hara M, Björkbom A, et al. Critical differences in drug metabolic properties of human hepatic cellular models, including primary human hepatocytes, stem cell derived hepatocytes, and hepatoma cell lines. Biochemical Pharmacol. 2018;155:124–40.
Adam AAA, van der Mark VA, Donkers JM, Wildenberg ME, Oude Elferink RPJ, Chamuleau RAFM, et al. A practice-changing culture method relying on shaking substantially increases mitochondrial energy metabolism and functionality of human liver cell lines. PLoS ONE. 2018;13:e0193664.
Stephenne X, Najimi M, Ngoc DK, Smets F, Hue L, Guigas B, et al. Cryopreservation of human hepatocytes alters the mitochondrial respiratory chain complex 1. Cell Transplant. 2007;16:409–19.
Matthew HWT, Sternberg J, Stefanovich P, Morgan JR, Toner M, Tompkins RG, et al. Effects of plasma exposure on cultured hepatocytes - implications for bioartificial liver support. Biotechnol Bioeng. 1996;51:100–11.
Stefanovich P, Matthew HWT, Toner M, Tompkins RG, Yarmush ML. Extracorporeal plasma perfusion of cultured hepatocytes - effect of intermittent perfusion on hepatocyte function and morphology. J Surg Res. 1996;66:57–63.
van Wenum M, Treskes P, Adam AAA, van der Mark VA, Jongejan A, Moerland PD, et al. HepaRG-progenitor cell derived hepatocytes cultured in bioartificial livers are protected from healthy- and acute liver failure-plasma induced toxicity. Cell Physiol Biochem. 2018;48:2189–204.
Zhao LF, Pan XP, Li LJ. Key challenges to the development of extracorporeal bioartificial liver support systems. Hepatobiliary Pancreat Dis Int. 2012;11:243–9.
Selden C, Bundy J, Erro E, Puschmann E, Miller M, Kahn D, et al. A clinical-scale BioArtificial Liver, developed for GMP, improved clinical parameters of liver function in porcine liver failure. Sci Rep. 2017;7:14518.
Qin G, Shao JG, Wang B, Shen Y, Zheng J, Liu XJ, et al. Artificial liver support system improves short- and long-term outcomes of patients with HBV-associated acute-on-chronic liver failure: a single-center experience. Medicine. 2014;93:e338.
Larsen FS, Schmidt LE, Bernsmeier C, Rasmussen A, Isoniemi H, Patel VC, et al. High-volume plasma exchange in patients with acute liver failure: an open randomised controlled trial. J Hepatol. 2016;64:69–78.
Larsen FS. Artificial liver support in acute and acute-on-chronic liver failure. Curr Opin Crit Care. 2019;25:187–91.
Ambrosino G, Varotto S, Basso SM, Cecchetto A, Carraro P, Naso A, et al. Hepatocyte transplantation in the treatment of acute liver failure: microencapsulated hepatocytes versus hepatocytes attached to an autologous biomatrix. Cell Transplant. 2003;12:43–9.
Maruyama M, Totsugawa T, Kunieda T, Okitsu T, Shibata N, Takesue M, et al. Hepatocyte isolation and transplantation in the pig. Cell Transplant. 2003;12:593–8.
Totsugawa T, Yong C, Rivas-Carrillo JD, Soto-Gutierrez A, Navarro-Alvarez N, Noguchi H, et al. Survival of liver failure pigs by transplantation of reversibly immortalized human hepatocytes with Tamoxifen-mediated self-recombination. J Hepatol. 2007;47:74–82.
Squires JE, Soltys KA, McKiernan P, Squires RH, Strom SC, Fox IJ, et al. Clinical hepatocyte transplantation: what is next? Curr Transplant Rep. 2017;4:280–9.
Bilir BM, Guinette D, Karrer F, Kumpe DA, Krysl J, Stephens J, et al. Hepatocyte transplantation in acute liver failure. Liver Transplant. 2000;6:32–40.
Lee CA, Sinha S, Fitzpatrick E, Dhawan A. Hepatocyte transplantation and advancements in alternative cell sources for liver-based regenerative medicine. J Mol Med. 2018;96:469–81.
Cao H, Yang J, Yu J, Pan Q, Li J, Zhou P, et al. Therapeutic potential of transplanted placental mesenchymal stem cells in treating Chinese miniature pigs with acute liver failure. BMC Med. 2012;10:56.
Li J, Zhang L Fau - Xin J, Xin J Fau - Jiang L, Jiang L Fau - Li J, Li J Fau - Zhang T, Zhang T Fau - Jin L, et al. Immediate intraportal transplantation of human bone marrow mesenchymal stem cells prevents death from fulminant hepatic failure in pigs. Hepatol. 2012;3:1044–52.
Zhu W, Shi XL, Xiao JQ, Gu GX, Ding YT, Ma ZL. Effects of xenogeneic adipose-derived stem cell transplantation on acute-on-chronic liver failure. Hepatobiliary Pancreat Dis Int. 2013;12:60–7.
Sang JF, Shi XL, Han B, Huang T, Huang X, Ren HZ, et al. Intraportal mesenchymal stem cell transplantation prevents acute liver failure through promoting cell proliferation and inhibiting apoptosis. Hepatobiliary Pancreat Dis Int. 2016;15:602–11.
Shi D, Zhang J, Zhou Q, Xin J, Jiang J, Jiang L, et al. Quantitative evaluation of human bone mesenchymal stem cells rescuing fulminant hepatic failure in pigs. Gut. 2017;66:955–64.
Lin NC, Wu HH, Ho JH, Liu CS, Lee OK. Mesenchymal stem cells prolong survival and prevent lethal complications in a porcine model of fulminant liver failure. Xenotransplant. 2019:e12542.
Lombardo E, van der Poll T, DelaRosa O, Dalemans W. Mesenchymal stem cells as a therapeutic tool to treat sepsis. World J Stem Cells. 2015;7:368–79.
Xu WX, He HL, Pan SW, Chen YL, Zhang ML, Zhu S, et al. Combination Treatments of Plasma Exchange and Umbilical Cord-Derived Mesenchymal Stem Cell Transplantation for Patients with Hepatitis B Virus-Related Acute-on-Chronic Liver Failure: A Clinical Trial in China. Stem Cells Int. 2019;2019:4130757.
Lin BL, Chen JF, Qiu WH, Wang KW, Xie DY, Chen XY, et al. Allogeneic Bone Marrow-Derived Mesenchymal Stromal Cells for HBV-Related Acute-on-Chronic Liver Failure: A Randomized Controlled Trial. Hepatol. 2017;66:209–19.
Sen S, Rose C, Ytrebo LM, Davies NA, Nedredal GI, Drevland SS, et al. Effect of albumin dialysis on intracranial pressure increase in pigs with acute liver failure: a randomized study. Crit Care Med. 2006;34:158–64.
Rose C, Ytrebo LM, Davies NA, Sen S, Nedredal GI, Belanger M, et al. Association of reduced extracellular brain ammonia, lactate, and intracranial pressure in pigs with acute liver failure. Hepatol. 2007;46:1883–922.
Ryska M, Laszikova E, Pantoflicek T, Ryska O, Prazak J, Koblihova E. Fractionated Plasma Separation and Adsorption Significantly Decreases Intracranial Pressure in Acute Liver Failure: Experimental Study. Eur Surg Res. 2009;42:230–5.
Al-Chalabi A, Matevossian E, Thaden V, Luppa P, Neiss A, Schuster T, et al. Evaluation of the Hepa Wash(R) treatment in pigs with acute liver failure. BMC Gastroenterol. 2013;13:83.
Laszikova E, Prazak J, Ryska O, Koblihova E, Tyll T, Ryska M. Fractionated plasmatic separation and adsorption does not alter haemodynamic parameters in experimental acute liver failure. Neuro Endocrinol Lett. 2014;35:280–4.
Lee KC, Baker LA, Stanzani G, Alibhai H, Chang YM, Jimenez Palacios C, et al. Extracorporeal liver assist device to exchange albumin and remove endotoxin in acute liver failure: Results of a pivotal pre-clinical study. J Hepatol. 2015;63:634–42.
Kortgen A, Rauchfuss F, Gotz M, Settmacher U, Bauer M, Sponholz C. Albumin dialysis in liver failure: comparison of molecular adsorbent recirculating system and single pass albumin dialysis–a retrospective analysis. Ther Apher Dial. 2009;13:419–25.
Huber W, Henschel B, Schmid R, Al-Chalabi A. First clinical experience in 14 patients treated with ADVOS: a study on feasibility, safety and efficacy of a new type of albumin dialysis. BMC Gastroenterol. 2017;17:1–11.
Hanish SI, Stein DM, Scalea JR, Essien EO, Thurman P, Hutson WR, et al. Molecular adsorbent recirculating system effectively replaces hepatic function in severe acute liver failure. Ann Surg. 2017;266:677–84.
Fujiwara K, Abe R, Yasui S, Yokosuka O, Kato N, Oda S. High recovery rate of consciousness by high-volume filtrate hemodiafiltration for fulminant hepatitis. Hepatol Res. 2019;49:224–31.
Sussman NL, Gislason GT, Kelly JH. Extracorporeal liver support. Application to fulminant hepatic failure. J Clin Gastroenterol. 1994;18:320–4.
Flendrig LM, Calise F, Di Florio E, Mancini A, Ceriello A, Santaniello W, et al. Significantly improved survival time in pigs with complete liver ischemia treated with a novel bioartificial liver. Int J Artif Org. 1999;22:701–9.
Sosef MN, Abrahamse LSL, van de Kerkhove MP, Hartman R, Chamuleau R, van Gulik TM. Assessment of the AMC-bioartificial liver in the anhepatic pig. Transplant. 2002;73:204–9.
Khalili TM, Navarro A, Ting P, Kamohara Y, Arkadopoulos N, Solomon BA, et al. Bioartificial liver treatment prolongs survival and lowers intracranial pressure in pigs with fulminant hepatic failure. Artif Organs. 2001;25:566–70.
Chen XP, Xue YL, Li XJ, Zhang ZY, Li YL, Huang ZQ. Experimental research on TECA-I bioartificial liver support system to treat canines with acute liver failure. World J Gastroenterol. 2001;7:706–9.
Wang N, Tsuruoka S, Yamamoto H, Enosawa S, Omasa T, Sata N, et al. The bioreactor With CYP3A4- and glutamine synthetase-Introduced HepG2 cells: treatment of hepatic failure with diazepam overdosage. Artif Organs. 2005;29:681–4.
Enosawa S, Miyashita T, Saito T, Omasa T, Matsumura T. The significant improvement of survival times and pathological parameters by bioartificial liver with recombinant HepG2 in porcine liver failure model. Cell Transplant. 2006;15:873–80.
Patzer JF, Lopez RC, Aggarwal S. Intracranial pressure observations in a canine model of acute liver failure supported by a bioartificial liver support system. Artif Organs. 2007;31:834–9.
Zhang Z, Zhao YC, Cheng Y, Jian GD, Pan MX, Gao Y. Hybrid bioartificial liver support in cynomolgus monkeys with D-galactosamine-induced acute liver failure. World J Gastroenterol. 2014;20:17399–406.
Glorioso JM, Mao SA, Rodysill B, Mounajjed T, Kremers WK, Elgilani F, et al. Pivotal preclinical trial of the spheroid reservoir bioartificial liver. J Hepatol. 2015;63:388–98.
Figaro S, Pereira U, Rada H, Semenzato N, Pouchoulin D, Legallais C. Development and validation of a bioartificial liver device with fluidized bed bioreactors hosting alginate-encapsulated hepatocyte spheroids. Conf Proc IEEE Eng Med Biol Soc. 2015;2015:1335–8.
Shi XL, Gao Y, Yan Y, Ma H, Sun L, Huang P, et al. Improved survival of porcine acute liver failure by a bioartificial liver device implanted with induced human functional hepatocytes. Cell Res. 2016;26:206–16.
Zhou P, Shao L, Zhao L, Lv G, Pan X, Zhang A, et al. Efficacy of fluidized bed bioartificial liver in treating fulminant hepatic failure in pigs: a metabolomics study. Sci Rep. 2016;6:26070.
Lee JH, Lee DH, Lee S, Kwon CHD, Ryu JN, Noh JK, et al. Functional evaluation of a bioartificial liver support system using immobilized hepatocyte spheroids in a porcine model of acute liver failure. Sci Rep. 2017;7:3804.
Li Y, Wu Q, Wang Y, Weng C, He Y, Gao M, et al. Novel spheroid reservoir bioartificial liver improves survival of nonhuman primates in a toxin-induced model of acute liver failure. Theranostics. 2018;8:5562–74.
Chen HS, Joo DJ, Shaheen M, Li Y, Wang Y, Yang J, et al. Randomized trial of spheroid reservoir bioartificial liver in porcine model of posthepatectomy liver failure. Hepatol. 2019;69:329–42.
Xue YL, Zhao SF, Yun L, Li XJ, Duan ZP, Chen XP, et al. TECA hybrid artificial liver support system in treatment of acute liver failure. World J Gastroenterol. 2001;7:826–9.
Mazariegos GV, Patzer JF, Lopez RC, Giraldo M, Devera ME, Grogan TA, et al. First clinical use of a novel bioartificial liver support system (BLSS). Am J Transplant. 2002;2:260–6.
van de Kerkhove MP, Poyck PP, Deurholt T, Hoekstra R, Chamuleau RA, van Gulik TM. Liver support therapy: an overview of the AMC-bioartificial liver research. Dig Surg. 2005;22:254–64.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Chamuleau, R.A.F.M., Hoekstra, R. End-stage liver failure: filling the treatment gap at the intensive care unit. J Artif Organs 23, 113–123 (2020). https://doi.org/10.1007/s10047-019-01133-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10047-019-01133-3