Abstract
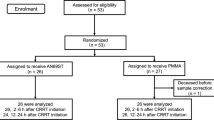
Cardiopulmonary bypass (CPB) induces a complex inflammatory response involving an increase in inflammatory cytokines, called postperfusion syndrome. Previous studies demonstrated that adsorption of the serum cytokines can reduce acute inflammation and improve clinical outcomes. In this study, patients were placed on continuous renal replacement therapy (CRRT) with a polymethyl methacrylate (PMMA) membrane hemofilter immediately after the start of an open-heart surgery with CPB and throughout the postoperative course to prevent postperfusion syndrome. The aim of this study was to assess whether continuous CRRT using a PMMA filter (PMMA–CRRT) could affect cytokine expression and improve perioperative outcomes. We designed a randomized controlled trial, which included 19 consecutive adult patients on maintenance dialysis and 7 consecutive adult patients who were not on maintenance dialysis (NHD group). Patients on maintenance dialysis were randomly divided into two groups: Ten patients who received CRRT with a polysulfone membrane hemofilter (PS group) and nine patients who received CRRT with a PMMA membrane (PMMA group). Blood samples were collected from the radial or brachial artery at five different time points. Comparisons between the PS, PMMA, and NHD groups revealed a significant main effect of time on changes in serum IL-6 and IL-8 concentrations (p < 0.01) and an interaction (p < 0.05) between time and group. Plasma IL-6 and IL-8 levels after surgery were significantly lower in the PMMA group than in the PS group, while other cytokines measured in this study were not significantly different. In addition, clinical outcomes were not significantly different between the groups. The continuous use of PMMA-CRRT throughout the perioperative period suppressed serum IL-6 and IL-8 concentrations, although there were no differences in clinical outcomes.

Similar content being viewed by others
References
Westaby S. Organ dysfunction after cardiopulmonary bypass. A systemic inflammatory reaction initiated by the extracorporeal circuit. Intensive Care Med. 1987;13(2):89–95.
Kawamura T, Wakusawa R, Okada K, Inada S. Evaluations of cytokines during open heart surgery with cardiopulmonary bypass: participation of interleukin 8 and 6 in reperfusion injury. Can J Anaesth. 1993;40:1016–21.
Sawa Y, Shimazaki Y, Kadoba K, Masai T, Fukuda H, Ohata T, et al. Attenuation of cardiopulmonary bypass derived inflammatory reactions reduces myocardial reperfusion injury in open heart surgery. J Thorac Cardiovasc Surg. 1996;111:29–35.
Engels M, Bilgic E, Pinto A, Vasquez E, Wollschläger L, Steinbrenner H, et al. A cardiopulmonary bypass with deep hypothermic circulatory arrest rat model for the investigation of the systemic inflammation response and induced organ damage. J Inflamm. 2014;12:11: 26.
Kimura F, Shimizu H, Yoshidome H, Ohtsuka M, Miyazaki M. Immunosuppression following surgical and traumatic injury. Surg Today. 2010;40(9):793–808.
Wan S, LeClerc JL, Vincent JL. Inflammatory response to cardiopulmonary bypass: mechanisms involved and possible therapeutic strategies. Chest. 1997;112:676–92.
Moat NE, Shore DF, Evans TW. Organ dysfunction and cardiopulmonary bypass: the role of complement and complement regulatory proteins. Eur J Cardiothorac Surg. 1993;7:563–73.
Kirklin JK, Westaby S, Blackstone EH, Kirklin JW, Chenoweth DE, Pacifico AD. Complement and the damaging effects of cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1983;86:845–57.
Steinberg J, Fink G, Picone A, Searles B, Schiller H, Lee HM, et al. Evidence of increased matrix metalloproteinase-9 concentration in patients following cardiopulmonary bypass. J Extra Corpor Technol. 2001;33(4):218 – 22.
Prasad A, Stone GW, Holmes DR, Gersh B. Reperfusion injury, microvascular dysfunction, and cardioprotection: the dark side of reperfusion. Circulation. 2009;120(21):2105–12.
Sanada S, Komuro I, Kitakaze M. Pathophysiology of myocardial reperfusion injury: preconditioning, postconditioning, and translational aspects of protective measures. Am J Physiol Heart Circ Physiol. 2011;301(5):H1723-H1741.
Maganti M, Badiwala M, Sheikh A, Scully H, Feindel C, David TE, et al. Predictors of low cardiac output syndrome after isolated mitral valve surgery. J Thorac Cardiovasc Surg. 2010;140(4):790–6.
Stenvinkel P, Heimbürger O, Lindholm B, Kaysen GA, Bergström J. Are there two types of malnutrition in chronic renal failure? Evidence for relationships between malnutrition, inflammation, and atherosclerosis (MIA syndrome). Nephrol Dial Transplant. 2000;15:953–60.
Herbelin A, Ureña P, Nguyen AT, Zingraff J, Descamps-Latscha B. Elevated circulating levels of interleukin-6 in patients with chronic renal failure. Kidney Int. 1991;39(5):954 – 60.
Shann KG, Likosky DS, Murkin JM, Baker RA, Baribeau YR, DeFoe GR, et al. An evidence-based review of the practice of cardiopulmonary bypass in adults: a focus on neurologic injury, glycemic control, hemodilution, and the inflammatory response. J Thorac Cardiovasc Surg. 2006; 132(2):283 – 90.
Jiménez JJ, Iribarren JL, Brouard M, Hernández D, Palmero S, Jiménez A, et al. Safety and Effectiveness of two treatment regimes with tranexamic acid to minimize inflammatory response in elective cardiopulmonary bypass patients: a randomized double-blind, dosedependent, phase IV clinical trial. J Cardiothorac Surg. 2011;14:6: 138.
Zhang X, Zhou C, Zhuang J, Xiao X, Zheng S, Xiong W, et al. Effects of leukocyte depletion on cardiopulmonary protection and inflammation after valve surgery. Int J Artif Organs. 2010;33(11):812–8.
Blomquist S, Gustafsson V, Manolopoulos T, Pierre L. Clinical experience with a novel endotoxin adsorbtion device in patients undergoing cardiac surgery. Perfusion. 2009;24(1):13–7.
Pathan N, Hemingway CA, Alizadeh AA, Stephens AC, Boldrick JC, Oragui EE, et al. Role of interleukin 6 in myocardial dysfunction of meningococcal septic shock. Lancet. 2004;363(9404):203–9.
Pathan N, Franklin JL, Eleftherohorinou H, Wright VJ, Hemingway CA, Waddell SJ, et al. Myocardial depressant effects of interleukin 6 in meningococcal sepsis are regulated by p38 mitogen-activated protein kinase. Crit Care Med. 2011;39(7):1692–711.
Stefoni S, La Manna G, Zanchelli F, Dalmastri V, Perna C, Pace G, et al. Clinical biology of artificial organ substitution. Nephrol Dial Transplant. 1998;13(Suppl 7):51–4.
Heidland A, Sebekova K, Schinzel R. Advanced glycation end products and the progressive course of renal disease. Am J Kidney Dis. 2001;38:S100–S106.
Panihi V, Miglion M, De Pietro S, Taccola D, Bianchi AM, Giovannini L, et al. C-reactive protein and interleukin-6 level are related to renal function in predialytic chronic renal failure. Nephron. 2002;91:594–600.
Halter J, Steinberg J, Fink G, Lutz C, Picone A, Maybury R, et al. Evidence of systemic cytokine release in patients undergoing cardiopulmonary bypass. J Extra Corpor Technol. 2005;37(3):272–7.
Bellomo R, Tipping P, Boyce N. Continuous veno-venous hemofiltration with dialysis removes cytokines from the circulation of septic patients. Crit Care Med. 1993;21:522–26.
Hirayama Y, Oda S, Tateishi Y, Sadahiro T, Nakamura M, Watanabe E, et al. Comparison of interleukin-6 removal properties among hemofilters consisting of varying membrane materials and surface areas: An in vitro study. Blood Purif. 2011;31:18–25.
Nakada T, Oda S, Hirosawa H, Sadahiro T, Nakamura M, Abe R, et al. Continuous hemodiafiltration with PMMA hemofilter in the treatment of patients with septic shock. Mol Med. 2008;14:257–63.
Matsuda K, Moriguchi T, Harii N, Goto J. Comparison of efficacy between continuous hemodiafiltration with a PMMA membrane hemofilter and a PAN membrane hemofilter in the treatment of a patient with septic acute renal failure. Transfus Apher Sci. 2009;40:49–53.
Faubel S, Edelstein CL. Mechanisms and mediators of lung injury after acute kidney injury. Nat Rev Nephrol. 2016;12(1):48–60.
Yimin H, Wenkui Y, Jialiang S, Qiyi C, Juanhong S, Zhiliang L, et al. Effects of continuous renal replacement therapy on renal inflammatory cytokines during extracorporeal membrane oxygenation in a porcine model. J Cardiothorac Surg. 2013;29:8: 113.
Shi J, Chen Q, Yu W, Shen J, Gong J, He C, et al. Continuous renal replacement therapy reduces the systemic and pulmonary inflammation induced by venovenous extracorporeal membrane oxygenation in a porcine model. Artif Organs. 2014;38(3):215 – 23.
Liu KD, Himmelfarb J, Paganini E, Ikizler TA, Soroko SH, Mehta RL, et al. Timing of initiation of dialysis in critically ill patients with acute kidney injury. Clin J Am Soc Nephrol. 2006;1(5):915–9.
Bagshaw SM, Uchino S, Bellomo R, Morimatsu H, Morgera S, Schetz M, et al. Timing of renal replacement therapy and clinical outcomes in critically ill patients with severe acute kidney injury. J Crit Care. 2009;24(1):129 – 40.
Acknowledgements
We thank all the staff for their assistance in conducting this study. This research received no specific Grants from any funding agency in the public, commercial, or not-for-profit sectors.
Funding
This research received no specific Grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Mukaida, H., Matsushita, S., Inotani, T. et al. Continuous renal replacement therapy with a polymethyl methacrylate membrane hemofilter suppresses inflammation in patients after open-heart surgery with cardiopulmonary bypass. J Artif Organs 21, 188–195 (2018). https://doi.org/10.1007/s10047-018-1025-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10047-018-1025-6